stacylover
Slaying stacys
- Joined
- Aug 13, 2025
- Posts
- 354
- Reputation
- 341
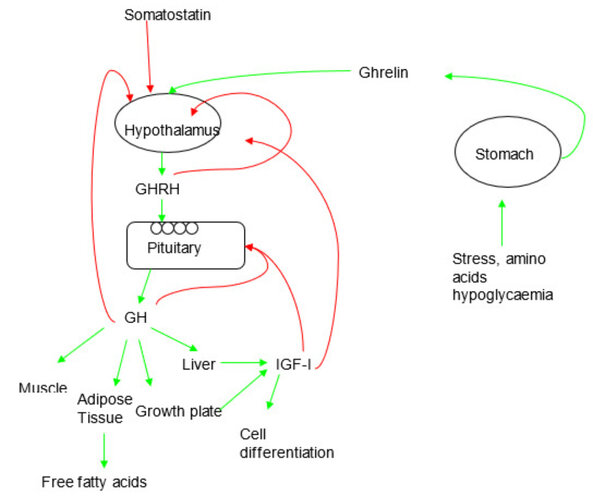
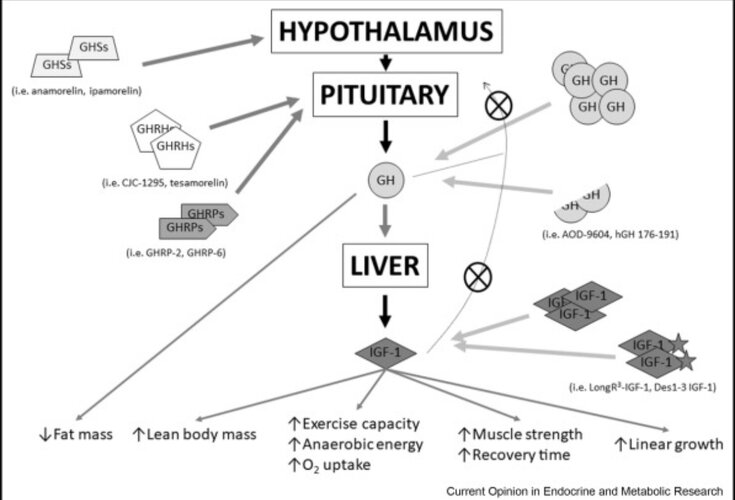
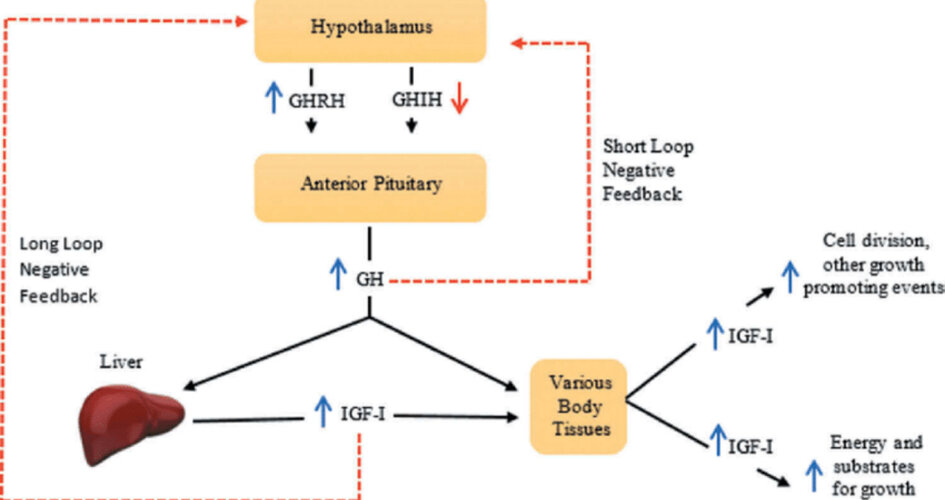
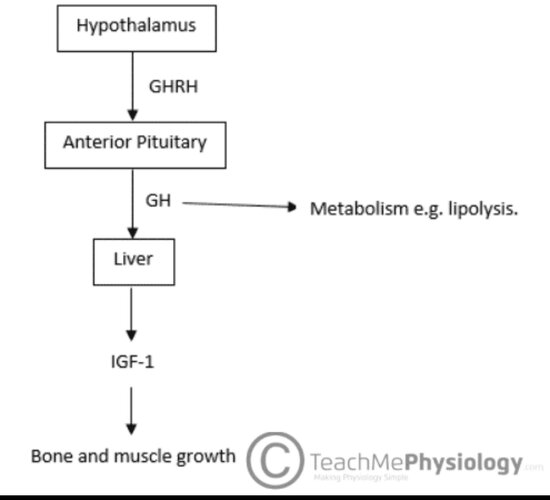
CJC-1295 is a synthetic analogue of Growth Hormone Releasing Hormone (GHRH). Its function is to stimulate the pituitary gland to release growth hormone (GH), which in turn elevates insulin-like growth factor 1 (IGF-1) levels. IGF-1 is the primary mediator of bone elongation, cartilage development, and muscle growth.
There are two forms of CJC-1295:
The hypothesis is that artificially increasing GH and IGF-1 levels during puberty could enhance height growth. While this is theoretically possible, the scientific evidence does not support it in healthy adolescents. Puberty naturally produces high levels of GH and IGF-1, which drive rapid growth. Ultimately, height potential is limited by genetics and the closure of growth plates (epiphyseal plates). Once these plates close, further height increase is impossible.
To date, there are no peer-reviewed clinical studies demonstrating that CJC-1295, with or without DAC, increases height in healthy adolescents. The peptide has mainly been studied in adults, particularly older men or patients with GH deficiency, where it was shown to raise GH and IGF-1 levels but not to extend stature.
Anecdotal reports, such as those on online forums, claim very small increases in height when CJC-1295 No DAC is used in combination with other peptides (for example, ipamorelin). However, these claims are unverified and cannot be considered reliable scientific evidence.
3. Risks and Side Effects
Reported side effects of CJC-1295 include injection site reactions (redness, swelling, itching), headaches, nausea, flushing, fatigue, dizziness, and irritability. Overstimulation of GH may disrupt the endocrine system, potentially leading to abnormal tissue growth, insulin resistance, or acromegaly-like features if used improperly.
A further concern is hormonal imbalance. Introducing exogenous stimulation of GH release may interfere with natural GH regulation and reduce the body’s own secretion through negative feedback mechanisms. In adolescents, this could disrupt the delicate balance of hormones during puberty.
4. Legal and Medical Status
CJC-1295 is not approved for medical use in children or adolescents. It is typically classified as a research chemical rather than a medication. The only approved medical treatment for growth delay or GH deficiency in children is recombinant human growth hormone (hGH), which must be prescribed and supervised by a pediatric endocrinologist.
5. Summary
CJC-1295 No DAC does stimulate growth hormone release in a pulsatile pattern that resembles natural physiology. However, there is no scientific evidence that it increases final height in healthy adolescents undergoing normal puberty. The potential benefits are theoretical, while the risks—hormonal disruption, side effects, and legal issues—are real.
The current medical consensus is clear: if growth or height is a concern, the appropriate and safe approach is to consult a pediatric endocrinologist. Only under medical supervision and after proper diagnostic testing can growth hormone therapy be considered, and only in cases of verified growth hormone deficiency or related medical conditions.
There are two forms of CJC-1295:
- CJC-1295 with DAC (Drug Affinity Complex): Has a long half-life (6–8 days), producing prolonged GH and IGF-1 elevation.
- CJC-1295 without DAC (No DAC): Has a much shorter half-life (30 minutes to 2 hours), producing short-lived, pulsatile GH bursts. This pulsatile release is closer to the body’s natural secretion pattern of GH.
The hypothesis is that artificially increasing GH and IGF-1 levels during puberty could enhance height growth. While this is theoretically possible, the scientific evidence does not support it in healthy adolescents. Puberty naturally produces high levels of GH and IGF-1, which drive rapid growth. Ultimately, height potential is limited by genetics and the closure of growth plates (epiphyseal plates). Once these plates close, further height increase is impossible.
To date, there are no peer-reviewed clinical studies demonstrating that CJC-1295, with or without DAC, increases height in healthy adolescents. The peptide has mainly been studied in adults, particularly older men or patients with GH deficiency, where it was shown to raise GH and IGF-1 levels but not to extend stature.
Anecdotal reports, such as those on online forums, claim very small increases in height when CJC-1295 No DAC is used in combination with other peptides (for example, ipamorelin). However, these claims are unverified and cannot be considered reliable scientific evidence.
3. Risks and Side Effects
Reported side effects of CJC-1295 include injection site reactions (redness, swelling, itching), headaches, nausea, flushing, fatigue, dizziness, and irritability. Overstimulation of GH may disrupt the endocrine system, potentially leading to abnormal tissue growth, insulin resistance, or acromegaly-like features if used improperly.
A further concern is hormonal imbalance. Introducing exogenous stimulation of GH release may interfere with natural GH regulation and reduce the body’s own secretion through negative feedback mechanisms. In adolescents, this could disrupt the delicate balance of hormones during puberty.
4. Legal and Medical Status
CJC-1295 is not approved for medical use in children or adolescents. It is typically classified as a research chemical rather than a medication. The only approved medical treatment for growth delay or GH deficiency in children is recombinant human growth hormone (hGH), which must be prescribed and supervised by a pediatric endocrinologist.
5. Summary
CJC-1295 No DAC does stimulate growth hormone release in a pulsatile pattern that resembles natural physiology. However, there is no scientific evidence that it increases final height in healthy adolescents undergoing normal puberty. The potential benefits are theoretical, while the risks—hormonal disruption, side effects, and legal issues—are real.
The current medical consensus is clear: if growth or height is a concern, the appropriate and safe approach is to consult a pediatric endocrinologist. Only under medical supervision and after proper diagnostic testing can growth hormone therapy be considered, and only in cases of verified growth hormone deficiency or related medical conditions.



 madddd
madddd