RAITEIII
Legendary
- Joined
- Jun 20, 2019
- Posts
- 23,760
- Reputation
- 23,613
I want to convert 50 mg of a compound into micromolars (um).
Compound moral mass: 313.87
Compound moral mass: 313.87
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: this_feature_currently_requires_accessing_site_using_safari
Thanks a lot. Indeed I have not ever read any of the concepts. Is there anything you'd recommend me to get started to have at least basic knowledge? Perhaps I can do some online chemistry course.Moles = 0.05 g(50mg) / 313.87 g/mol = 0.0001593 mol
Micromoles = 0.0001593 mol * 10^6 = 159.3 μM
The question is pretty straight forward and easy. If you don't know this you probably haven't even read the basics of mole concepts tbh.
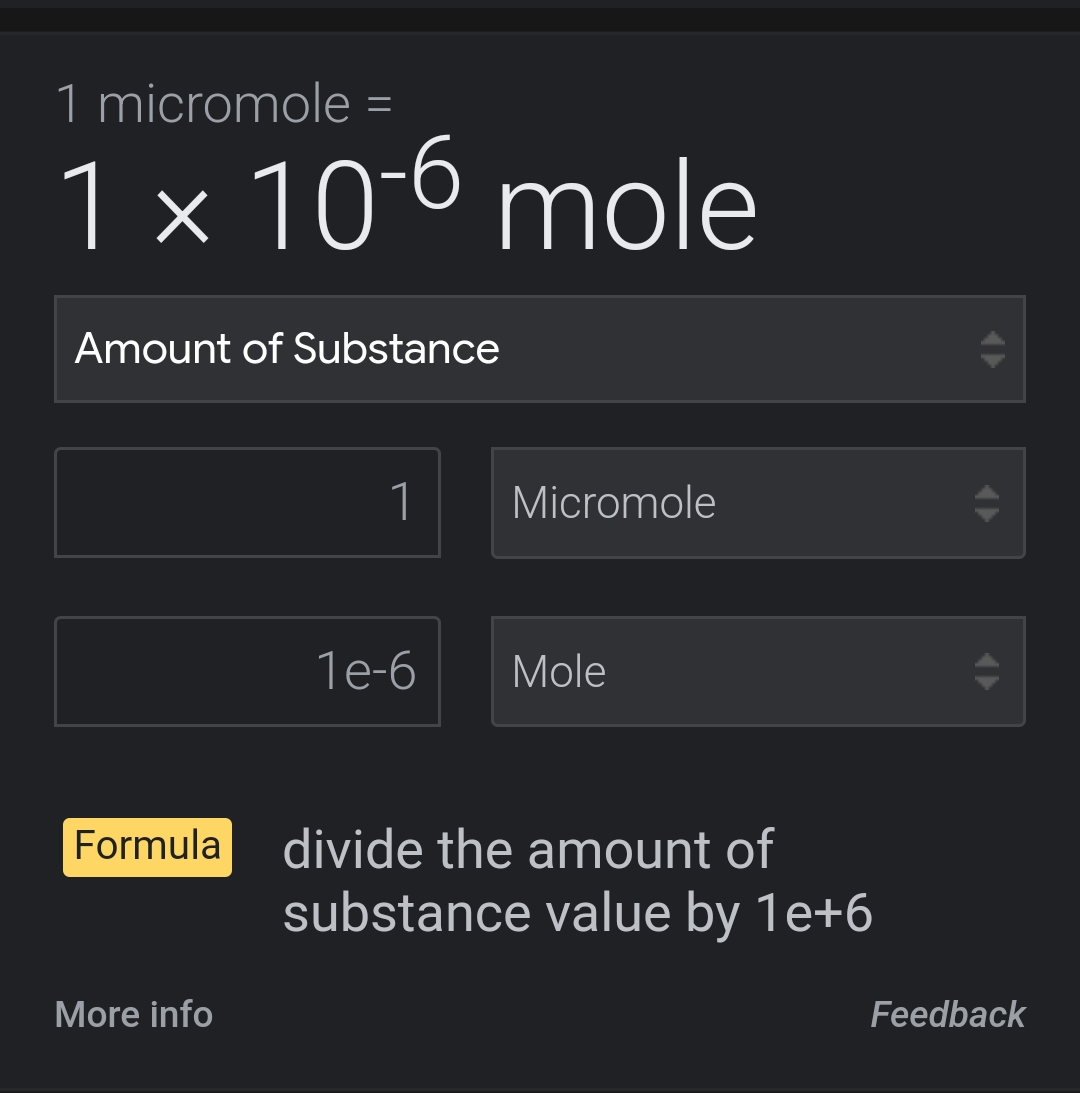
I'd divide moles / molar mass. Then the result (0.001593) is multiplied by 10^6. Why exactly 10^6? Will Google later after work.Moles = 0.05 g(50mg) / 313.87 g/mol = 0.0001593 mol
Micromoles = 0.0001593 mol * 10^6 = 159.3 μM
The question is pretty straight forward and easy. If you don't know this you probably haven't even read the basics of mole concepts tbh.
Idk till what level in chemistry your course requires but physical chemistry by Peter Atkins is pretty indepth and goes from the basics to the advanced concepts. Not many problems but it's good if you want to strengthen your concepts.Thanks a lot. Indeed I have not ever read any of the concepts. Is there anything you'd recommend me to get started to have at least basic knowledge? Perhaps I can do some online chemistry course.
Have a good day
Micromoles to molesI'd divide moles / molar mass. Then the result (0.001593) is multiplied by 10^6. Why exactly 10^6? Will Google later after work.