ihatemySOST
Bronze
- Joined
- Sep 5, 2025
- Posts
- 479
- Reputation
- 771
Hello, most of you have already seen my post about subperiosteal hematomas, and it was quite popular. However, some people think they've "debunked my theory," like @dookielooksmaxxer. He didn't actually debunk anything; he just said that calcification of the hematoma would be unsightly. So, he seems to agree with me that bone regeneration is indeed possible according to my theory, but he still showed some ridiculous examples.
Initially, he showed a picture of a child who had a subperiosteal hematoma "https://www.cureus.com/articles/64205-calvarium-subperiosteal-hematoma-in-a-12-year-old-boy#!/" where the hematoma occurred because he bumped his head against another player's head, resulting in a subperiosteal hematoma in the skull. The doctor then... The blood was aspirated using a needle, and it did not calcify, allowing the child to return to his normal life. A follow-up CT scan showed the complete disappearance of the subperiosteal hematoma on Day 57. The boy has returned to soccer-playing without sequelae. This case suggests that 1) C-SPOH can be found in healthy juveniles; 2) Neovascularization along the wall of the C-SPOH cavity may contribute to the formation of the C-SPOH; 3) A simple aspiration of the liquefied SPOH may fail to cure it in juveniles. Now, the reader might be wondering how this refutes my theory. Honestly, I don't know how. I think he means that the hematoma was random, and he assumed it was a bony mass even though it hadn't calcified at all. But I honestly don't know what to tell him. The blow was strong, random, and spread over a large surface, so the hematoma simply formed at the site of the blow, and then it would also calcify at the site of the blow (if it calcified, because not every hematoma (A calcified hematoma), but he posted this image without explaining anything; apparently, he didn't even read the study, because he thought the hematoma that appeared was a bony mass.
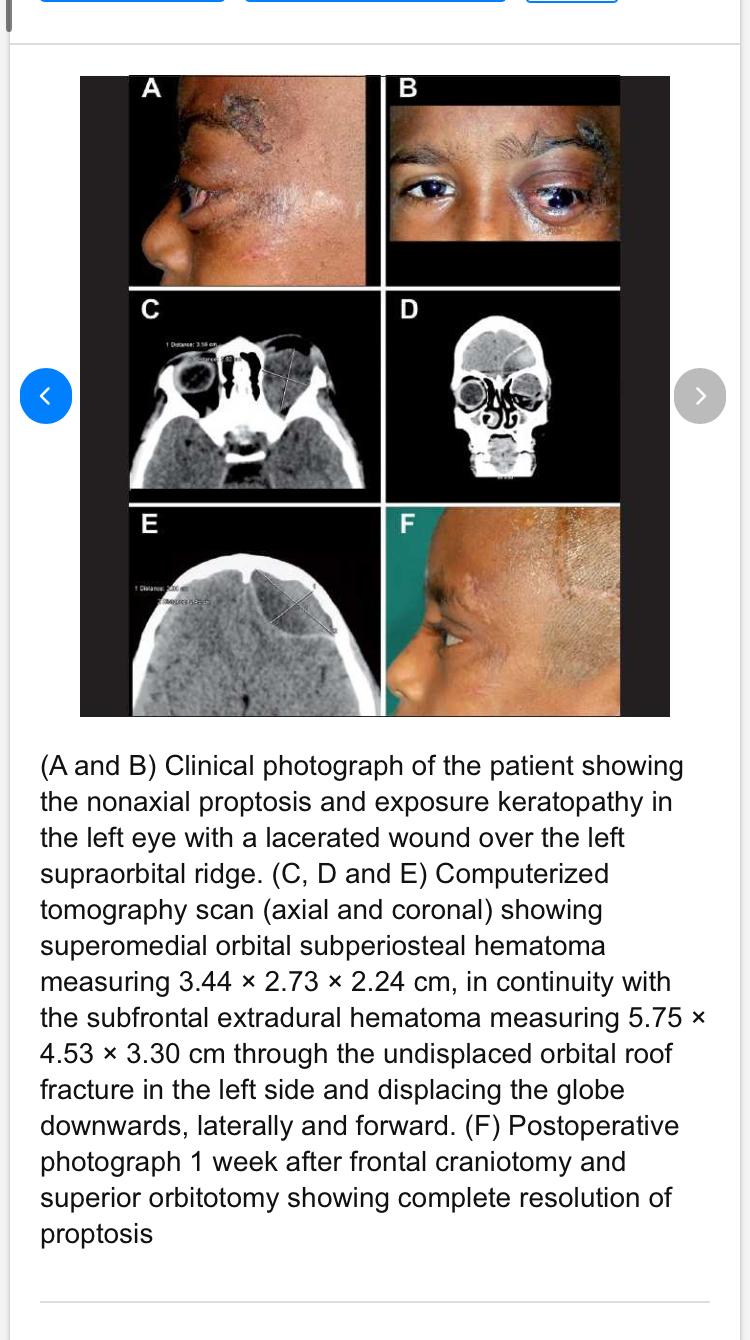
In the second image he brought, "https://www.researchgate.net/figure...nonaxial-proptosis-and-exposure_fig3_49682451", he brought a picture of a young child who had a hematoma in the orbit. Well, the cause was actually a fracture of the orbit (a complete fracture). Because of this fracture, the hematoma occurred, and this hematoma pushed the eye down and forward (and it started to look distorted). I think you're going to wonder again, "Does this contradict my theory?" I'll answer you: even I personally don't know. Perhaps he thinks the hematoma will be random, resulting in random calcification and an unsightly appearance. Well, that might be true if there's a random injury to the bone, or a random hematoma. It's natural for random calcification to occur. I mentioned this in my previous post; your body builds bone at the site of the hematoma (the injury site), and it doesn't It builds aesthetically. You control whether the construction will be aesthetic or random, and that's determined by your precise hitting. We can look at rodeo players, for example, where the bone only grows at the site of the hematoma, literally only there. Now, if you hit your cheekbone, nothing magical happens and the bone doesn't grow unaesthetically. The bone will grow at the point of impact, the site of the hematoma. The bone won't grow unaesthetically unless you're mentally challenged. If not, how do you explain the natural and precise bone growth in rodeo players? It's not just a random hematoma due to a complete bone fracture. Lol. Also, you said I agree with you that the bone won't be aesthetically pleasing, but I didn't say it won't be. I said who said it will be, but I didn't deny it. It might be, and it might not. It depends on the IQ of the person doing the smashing.
Also, even if we assume, based on your premise, that hematoma formation and calcification will magically occur randomly, I still don't understand how this refutes my point. Wasn't my post fundamentally about the potential effectiveness of bone-smashing through increased bone mass via subperiosteal hematoma formation, regardless of aesthetics? So this doesn't refute my main theory; it simply demonstrates that if you're stupid, growth will occur randomly.
Regarding the effect of hPTH(1-34) on bone formation, studies have shown that the rate of bone building increases significantly on the spongy, intrinsic cortical, and periosteal surfaces, and one study suggested that this might potentially lead to an increase in bone diameter. However, the reason for this cautious formulation by the researchers lies in the nature of the treatment-induced bone formation. The vast majority of new bone was a result of remodeling-based formation (RBF), that is, bone remodeling within the boundaries of pre-existing bone without net expansion (modeling). In this process, hPTH stimulates the resorption of old bone followed by rapid formation by osteoblasts, with subsequent cell death, resulting in high formation rates after a short period compared to controls. This intrinsic formation enhances bone density and cortical thickness but does not cause a significant increase in the external diameter of the bone.
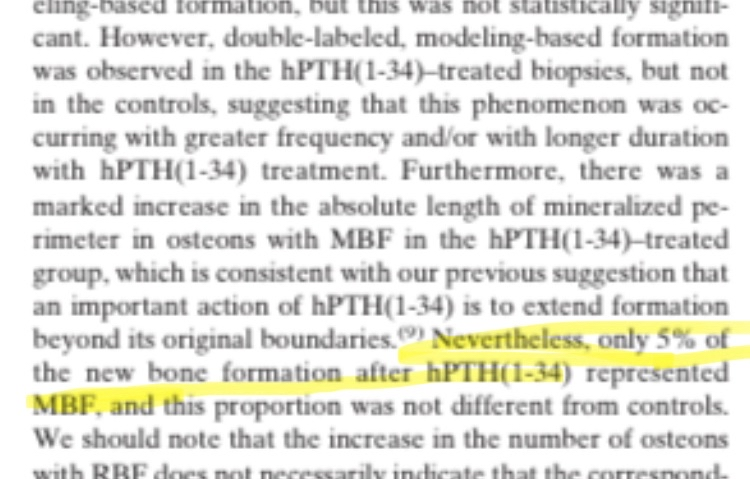
Modeling-Based Formation (MBF) is the formation of new bone on previously unabsorbed surfaces, representing net bone growth that can expand the bone and increase its diameter. The same study noted the presence of double-labeled MBF in biopsies of patients who received hPTH (1-34), while this was not observed in controls, suggesting that this type of formation occurs more frequently or over a longer period with treatment. A significant increase in the length of the mineralized perimeter was also observed in MBF-associated osteons, consistent with the hypothesis that hPTH can expand bone formation beyond its original boundaries. However, MBF constituted a very small proportion of the new bone (~5%), and this proportion did not differ from controls, confirming that most of the new bone came from RBF rather than MBF, and therefore the increase in the external diameter of the bone was not significant. This small percentage means that even with continued treatment, any real increase in diameter may take many years to become noticeable, and measurements on small biopsies (histomorphometry) cannot necessarily reveal the actual bone diameter increase, which can only be measured by high-resolution in vivo imaging techniques such as HR-pQCT.
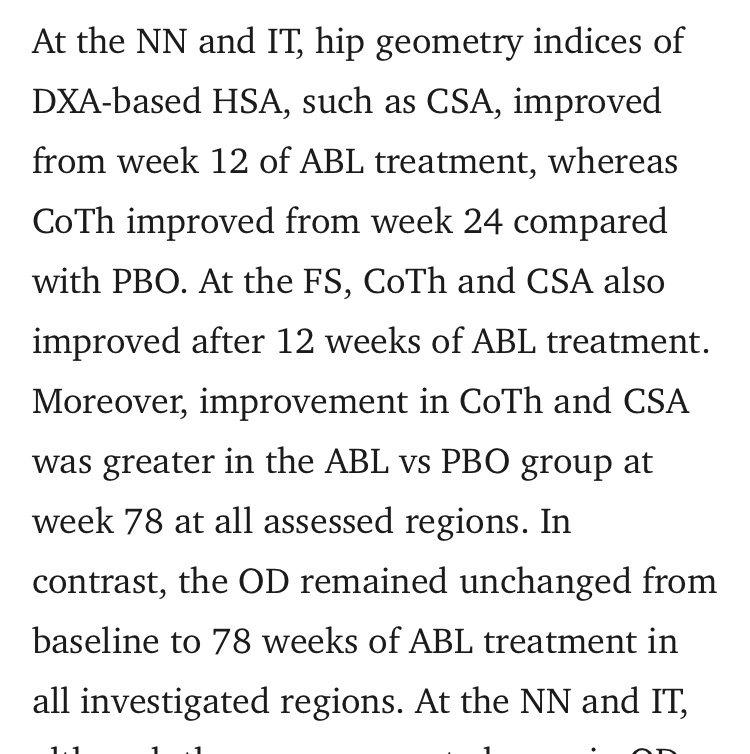
 To answer a question that scientists have debated for decadeswhether PTH analogs can increase bone diameter (OD)a recent, controlled, randomized, double-blind, placebo-controlled trial with a large number of participants and a duration of 18 months was conducted, making it ideal for reliably evaluating this question. The results showed that treatment with PTH analogs led to a significant increase in bone density, cortical thickness (CoTh), and cortical spongy bone volume (CSA) compared to the placebo. However, despite improvements in cortical thickness and cross-section, bone diameter (OD) did not increase significantly. This may seem paradoxical, but it actually reflects normal bone growth mechanisms. Increases in cortical thickness and cross-sectional volume occur primarily through endosteal formation from the inside out, toward the bone cavity, rather than outward. This results in increased bone volume and density without a significant expansion of the outer diameter. This is because PTH analogs primarily work through Remodeling-Based Formation (RBF), meaning bone remodeling within existing boundaries, rather than Modeling-Based Formation (MBF), which generates net growth that can increase the outer diameter. Therefore, any true expansion of the outer diameter (OD) may take many years to detect, even with the most precise measurement techniques. Thus, an increase in cortical volume (CSA) does not necessarily indicate an expansion of the outer bone, and any noticeable cortical expansion (appositional growth) depends on MBF, which accounts for a very small percentage of growth after treatment with PTH analogs. In summary, PTH analogs significantly enhance bone density and internal volume, but they don't substantially increase bone diameter in the short to medium term. This is because bone growth occurs primarily through internal remodeling, not external bone growth. Any real changes in OD require long-term monitoring using precise techniques. So, you haven't refuted my point; you've simply demonstrated your lack of understanding of the fundamentals of bone growth, which is frankly unfortunate.
To answer a question that scientists have debated for decadeswhether PTH analogs can increase bone diameter (OD)a recent, controlled, randomized, double-blind, placebo-controlled trial with a large number of participants and a duration of 18 months was conducted, making it ideal for reliably evaluating this question. The results showed that treatment with PTH analogs led to a significant increase in bone density, cortical thickness (CoTh), and cortical spongy bone volume (CSA) compared to the placebo. However, despite improvements in cortical thickness and cross-section, bone diameter (OD) did not increase significantly. This may seem paradoxical, but it actually reflects normal bone growth mechanisms. Increases in cortical thickness and cross-sectional volume occur primarily through endosteal formation from the inside out, toward the bone cavity, rather than outward. This results in increased bone volume and density without a significant expansion of the outer diameter. This is because PTH analogs primarily work through Remodeling-Based Formation (RBF), meaning bone remodeling within existing boundaries, rather than Modeling-Based Formation (MBF), which generates net growth that can increase the outer diameter. Therefore, any true expansion of the outer diameter (OD) may take many years to detect, even with the most precise measurement techniques. Thus, an increase in cortical volume (CSA) does not necessarily indicate an expansion of the outer bone, and any noticeable cortical expansion (appositional growth) depends on MBF, which accounts for a very small percentage of growth after treatment with PTH analogs. In summary, PTH analogs significantly enhance bone density and internal volume, but they don't substantially increase bone diameter in the short to medium term. This is because bone growth occurs primarily through internal remodeling, not external bone growth. Any real changes in OD require long-term monitoring using precise techniques. So, you haven't refuted my point; you've simply demonstrated your lack of understanding of the fundamentals of bone growth, which is frankly unfortunate.
Both links :https://sci-hub.vg/10.1359/jbmr.070104, https://pmc.ncbi.nlm.nih.gov/articles/PMC10687120/
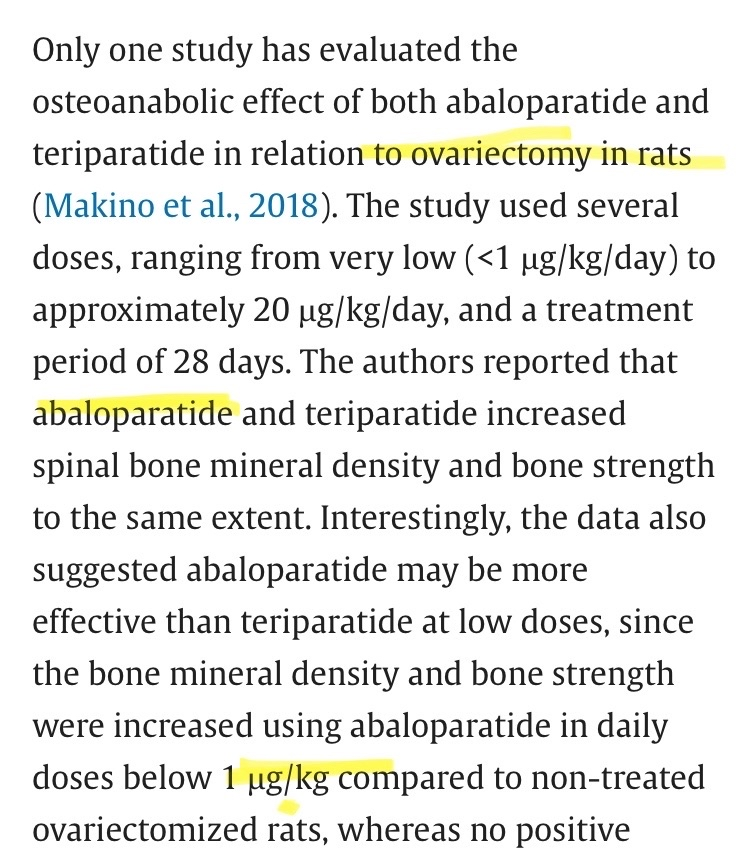
Now, to respond to your comment about my statement that the required dose to stimulate jaw growth in young rats is much higher than the standard doses, you replied that the bioavailability of subcutaneous ABL injection in male rats is much lower than in humans, as it was 38.9% in rats. However, in reality, the bioavailability in humans is even lower, at 36%. So, unfortunately, what you said here only proves one thing, which is that you asked chat gpt what the bioavailability is when injecting ABL subcutaneously in rats, and you didn't even research what it is in humans (which is very similar). Also, even if we assume that there is a slight difference in bioavailability, that doesn't change anything, because the doses that were successful in stimulating jaw growth were exclusively 8 mg/kg, and that was the only one that was successful, and any other dose was unsuccessful even though they are young rats, and these doses are hundreds of times higher than the standard doses. For the treatment of osteoporosis, the difference is not justified "through bioavailability." Furthermore, studies primarily conducted on rats do not differ in their results from humans; in rats, even very low doses of abl (1 mcg/kg) are sufficient to raise osteoporosis levels in old, ovariectomy-removed rats.

 But when it comes to stimulating mandibular growth in normal, growing rats, the dose required is approximately 8,000 times higher than the dose needed to increase bone density in older mice. What does this mean? It means that even if we assume that abl succeeded in increasing bone diameter or volume in humans at standard doses (which it certainly did not), the doses required to stimulate mandibular and facial bone growth in normal, growing adolescents could be hundreds of times higher than standard doses, and might not even be effective.
But when it comes to stimulating mandibular growth in normal, growing rats, the dose required is approximately 8,000 times higher than the dose needed to increase bone density in older mice. What does this mean? It means that even if we assume that abl succeeded in increasing bone diameter or volume in humans at standard doses (which it certainly did not), the doses required to stimulate mandibular and facial bone growth in normal, growing adolescents could be hundreds of times higher than standard doses, and might not even be effective.
B
Note : im not replying again if u didn’t present a good argument, and good reply about my pth analogs threads
Initially, he showed a picture of a child who had a subperiosteal hematoma "https://www.cureus.com/articles/64205-calvarium-subperiosteal-hematoma-in-a-12-year-old-boy#!/" where the hematoma occurred because he bumped his head against another player's head, resulting in a subperiosteal hematoma in the skull. The doctor then... The blood was aspirated using a needle, and it did not calcify, allowing the child to return to his normal life. A follow-up CT scan showed the complete disappearance of the subperiosteal hematoma on Day 57. The boy has returned to soccer-playing without sequelae. This case suggests that 1) C-SPOH can be found in healthy juveniles; 2) Neovascularization along the wall of the C-SPOH cavity may contribute to the formation of the C-SPOH; 3) A simple aspiration of the liquefied SPOH may fail to cure it in juveniles. Now, the reader might be wondering how this refutes my theory. Honestly, I don't know how. I think he means that the hematoma was random, and he assumed it was a bony mass even though it hadn't calcified at all. But I honestly don't know what to tell him. The blow was strong, random, and spread over a large surface, so the hematoma simply formed at the site of the blow, and then it would also calcify at the site of the blow (if it calcified, because not every hematoma (A calcified hematoma), but he posted this image without explaining anything; apparently, he didn't even read the study, because he thought the hematoma that appeared was a bony mass.

In the second image he brought, "https://www.researchgate.net/figure...nonaxial-proptosis-and-exposure_fig3_49682451", he brought a picture of a young child who had a hematoma in the orbit. Well, the cause was actually a fracture of the orbit (a complete fracture). Because of this fracture, the hematoma occurred, and this hematoma pushed the eye down and forward (and it started to look distorted). I think you're going to wonder again, "Does this contradict my theory?" I'll answer you: even I personally don't know. Perhaps he thinks the hematoma will be random, resulting in random calcification and an unsightly appearance. Well, that might be true if there's a random injury to the bone, or a random hematoma. It's natural for random calcification to occur. I mentioned this in my previous post; your body builds bone at the site of the hematoma (the injury site), and it doesn't It builds aesthetically. You control whether the construction will be aesthetic or random, and that's determined by your precise hitting. We can look at rodeo players, for example, where the bone only grows at the site of the hematoma, literally only there. Now, if you hit your cheekbone, nothing magical happens and the bone doesn't grow unaesthetically. The bone will grow at the point of impact, the site of the hematoma. The bone won't grow unaesthetically unless you're mentally challenged. If not, how do you explain the natural and precise bone growth in rodeo players? It's not just a random hematoma due to a complete bone fracture. Lol. Also, you said I agree with you that the bone won't be aesthetically pleasing, but I didn't say it won't be. I said who said it will be, but I didn't deny it. It might be, and it might not. It depends on the IQ of the person doing the smashing.

Also, even if we assume, based on your premise, that hematoma formation and calcification will magically occur randomly, I still don't understand how this refutes my point. Wasn't my post fundamentally about the potential effectiveness of bone-smashing through increased bone mass via subperiosteal hematoma formation, regardless of aesthetics? So this doesn't refute my main theory; it simply demonstrates that if you're stupid, growth will occur randomly.
Regarding the effect of hPTH(1-34) on bone formation, studies have shown that the rate of bone building increases significantly on the spongy, intrinsic cortical, and periosteal surfaces, and one study suggested that this might potentially lead to an increase in bone diameter. However, the reason for this cautious formulation by the researchers lies in the nature of the treatment-induced bone formation. The vast majority of new bone was a result of remodeling-based formation (RBF), that is, bone remodeling within the boundaries of pre-existing bone without net expansion (modeling). In this process, hPTH stimulates the resorption of old bone followed by rapid formation by osteoblasts, with subsequent cell death, resulting in high formation rates after a short period compared to controls. This intrinsic formation enhances bone density and cortical thickness but does not cause a significant increase in the external diameter of the bone.
Modeling-Based Formation (MBF) is the formation of new bone on previously unabsorbed surfaces, representing net bone growth that can expand the bone and increase its diameter. The same study noted the presence of double-labeled MBF in biopsies of patients who received hPTH (1-34), while this was not observed in controls, suggesting that this type of formation occurs more frequently or over a longer period with treatment. A significant increase in the length of the mineralized perimeter was also observed in MBF-associated osteons, consistent with the hypothesis that hPTH can expand bone formation beyond its original boundaries. However, MBF constituted a very small proportion of the new bone (~5%), and this proportion did not differ from controls, confirming that most of the new bone came from RBF rather than MBF, and therefore the increase in the external diameter of the bone was not significant. This small percentage means that even with continued treatment, any real increase in diameter may take many years to become noticeable, and measurements on small biopsies (histomorphometry) cannot necessarily reveal the actual bone diameter increase, which can only be measured by high-resolution in vivo imaging techniques such as HR-pQCT.



Both links :https://sci-hub.vg/10.1359/jbmr.070104, https://pmc.ncbi.nlm.nih.gov/articles/PMC10687120/
Now, to respond to your comment about my statement that the required dose to stimulate jaw growth in young rats is much higher than the standard doses, you replied that the bioavailability of subcutaneous ABL injection in male rats is much lower than in humans, as it was 38.9% in rats. However, in reality, the bioavailability in humans is even lower, at 36%. So, unfortunately, what you said here only proves one thing, which is that you asked chat gpt what the bioavailability is when injecting ABL subcutaneously in rats, and you didn't even research what it is in humans (which is very similar). Also, even if we assume that there is a slight difference in bioavailability, that doesn't change anything, because the doses that were successful in stimulating jaw growth were exclusively 8 mg/kg, and that was the only one that was successful, and any other dose was unsuccessful even though they are young rats, and these doses are hundreds of times higher than the standard doses. For the treatment of osteoporosis, the difference is not justified "through bioavailability." Furthermore, studies primarily conducted on rats do not differ in their results from humans; in rats, even very low doses of abl (1 mcg/kg) are sufficient to raise osteoporosis levels in old, ovariectomy-removed rats.


B
Note : im not replying again if u didn’t present a good argument, and good reply about my pth analogs threads
Last edited: