holy
- Joined
- Nov 5, 2024
- Posts
- 876
- Reputation
- 1,536
THREAD THEME
TABLE OF CONTENTS
SECTION | CONTENT |
|---|---|
I. Introduction: Why eye color is not a simple on/off switch | - The biology of iris pigmentation and light scattering - Why “brown vs blue” is a spectrum rather than a binary - Ancient myths vs molecular reality |
II. The genetic architecture of eye color | - Key genes: OCA2, HERC2, TYR, TYRP1, SLC24A4, SLC45A2, IRF4 - rs12913832: The enhancer SNP that basically flips the switch - How structural variation and regulatory networks create iris phenotypes |
III. The principle of somatic gene editing in the adult iris | - Why germline editing is irrelevant post-birth - Melanocyte biology and turnover rates - Challenges of altering a non-dividing cell population |
IV. Designing the edit: Tools and molecular strategies | - CRISPR-Cas9, CRISPRi, CRISPRa, and base/prime editing - Choosing the right Cas variant (SpCas9 vs SaCas9) - Target: Disrupting rs12913832 enhancer activity to mimic the blue-eyed genotype |
V. Vector delivery and ocular targeting | - AAV as the current gold standard - Payload size limits and promoter specificity - Injection strategies: Periocular, anterior chamber, or stromal delivery |
VI. Risks and physiological trade-offs | - Immune responses and off-target edits - Why reduced iris melanin increases UV damage risk - Partial heterochromia, light sensitivity, and long-term unknowns |
VII. Theoretical vs practical timelines of color change | - Why melanin degradation takes months - Uneven pigment remodeling and mosaic outcomes - Potential for reversibility (or lack thereof) |
VIII. Future directions: Precision editing and monitoring | - CRISPRi with reversible epigenetic switches - RNA base editing and prime editing for allele conversion |
IX. Conclusion | - What this technology reveals about the illusion of perfect self-modification (NOT just ranting here) - Why “genetic eye color change” is both possible and deeply impractical today |
SECTION I:
Introduction - Why eye color is not a simple on/off switch
Eye color isn’t controlled by a single “color gene.” It’s an emergent property of pigment density, structural scattering of light, and how the underlying iris tissues interact.
Historically, people assumed that blue eyes were simply “lighter” versions of brown, but molecular genetics revealed a complex regulatory network rather than a binary pigment switch.
People often think changing your eye color genetically is like swapping a paint color when you're literally interfering with a multi-gene regulatory network controlling pigment synthesis in a specific tissue, without altering the rest of your body’s pigmentation. It not only has to be precise, but also tissue-specific, and technically risky.
Historically, people assumed that blue eyes were simply “lighter” versions of brown, but molecular genetics revealed a complex regulatory network rather than a binary pigment switch.
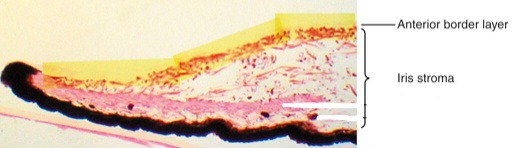
The iris has two main layers:
Anterior border layer + stroma

Where melanocytes reside. These cells produce melanin, mostly eumelanin (brown/black pigment) with little pheomelanin (red/yellow).
The amount of melanin in this layer determines how much light is absorbed.
Posterior pigmented epithelium

Almost always dark, regardless of your external eye color. It acts as a backdrop.
Blue eyes aren’t blue because they contain blue pigment. They lack dense stromal melanin, so light penetrates the stroma and scatters (the tyndall effect), making the iris appear blue, exactly how the sky looks blue despite being transparent atmosphere.
Brown eyes simply overwhelm this scattering with heavy eumelanin deposition.
Hazel and green are intermediate due to moderate melanin and some lipid-related scattering interactions.
Anterior border layer + stroma
Where melanocytes reside. These cells produce melanin, mostly eumelanin (brown/black pigment) with little pheomelanin (red/yellow).
The amount of melanin in this layer determines how much light is absorbed.
Posterior pigmented epithelium

Almost always dark, regardless of your external eye color. It acts as a backdrop.
Blue eyes aren’t blue because they contain blue pigment. They lack dense stromal melanin, so light penetrates the stroma and scatters (the tyndall effect), making the iris appear blue, exactly how the sky looks blue despite being transparent atmosphere.
Brown eyes simply overwhelm this scattering with heavy eumelanin deposition.
Hazel and green are intermediate due to moderate melanin and some lipid-related scattering interactions.
There’s no absolute switch that flips brown to blue. Instead, you have a continuum of pigmentation levels influenced by multiple genes. Even the “blue-eye” genotype doesn’t erase melanin completely, but just downregulates its synthesis enough for scattering to dominate.
This is why you can have grey-blue, steel-blue, or pale mixed hues depending on exact stromal density.
This is why you can have grey-blue, steel-blue, or pale mixed hues depending on exact stromal density.
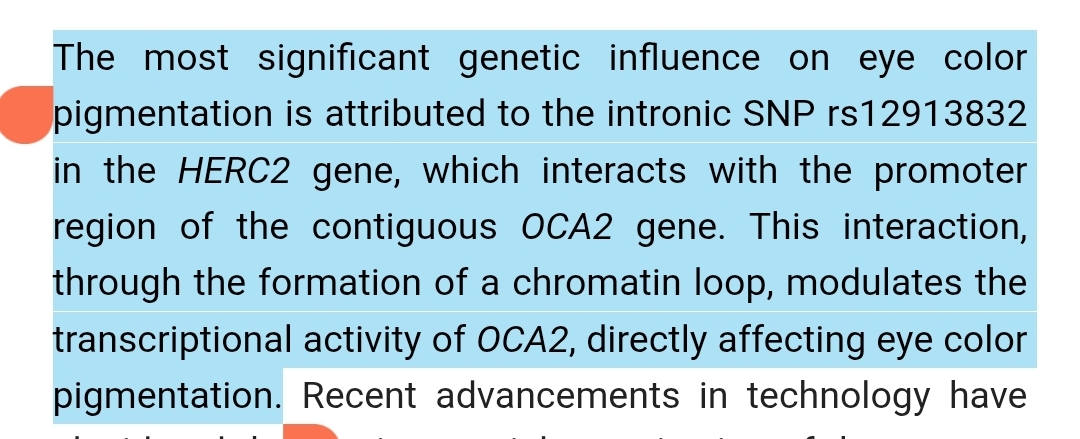
Ancient cultures often mythologized eye color, treating blue as mystical or rare. What they didn’t know is that most humans have the same structural base, but regulatory variations in melanin synthesis genes (particularly OCA2 controlled by an enhancer in HERC2) tip the balance.
A single SNP, rs12913832, dramatically reduces OCA2 transcription, lowering melanin production in iris melanocytes and shifting brown toward blue. But even that SNP’s effect depends on other background genes like TYR, SLC45A2, and IRF4, which fine-tune melanin metabolism.
A single SNP, rs12913832, dramatically reduces OCA2 transcription, lowering melanin production in iris melanocytes and shifting brown toward blue. But even that SNP’s effect depends on other background genes like TYR, SLC45A2, and IRF4, which fine-tune melanin metabolism.
People often think changing your eye color genetically is like swapping a paint color when you're literally interfering with a multi-gene regulatory network controlling pigment synthesis in a specific tissue, without altering the rest of your body’s pigmentation. It not only has to be precise, but also tissue-specific, and technically risky.
The study, titled “HERC2 rs12913832 modulates human pigmentation by attenuating OCA2 expression", provides direct mechanistic proof:

[1]
The study also describes allele‑specific chromatin looping between the enhancer region and the OCA2 promoter, mediated by transcription factors HLTF, LEF1, and MITF, offering molecular rationale for pigmentation differences.

[1]
The study also describes allele‑specific chromatin looping between the enhancer region and the OCA2 promoter, mediated by transcription factors HLTF, LEF1, and MITF, offering molecular rationale for pigmentation differences.
SECTION II:
The genetic architecture of eye color
Eye color arises from a polygenic system rather than a single determinant. Multiple loci interact to regulate how much melanin iris melanocytes synthesize and deposit.
To understand what you’re editing, you need to know which genes matter, how they interact, and why one SNP (rs12913832) dominates the phenotype.
To genetically induce a blue-eyed phenotype in a brown-eyed adult, you mimic the AA genotype at rs12913832 (HERC2) to downregulate OCA2 in iris melanocytes. But complete predictability is impossible without knowing the individual’s full polygenic background.
The main point I'm trying to make here is that rs12913832 in HERC2 is responsible for the vast majority (~68–80 %) of eye color variation between blue and brown. the rest of the variation, especially intermediate or unexpected phenotypes, comes from polygenic modifier effects in TYR, TYRP1, SLC24A4, IRF4, and others.
To understand what you’re editing, you need to know which genes matter, how they interact, and why one SNP (rs12913832) dominates the phenotype.
OCA2 (oculocutaneous albinism II)
Encodes the P protein that regulates pH and ion balance inside melanosomes, indirectly controlling melanin synthesis.
Higher OCA2 expression = more melanin = darker iris. Partial loss-of-function mutations lighten pigmentation.
HERC2
Doesn’t produce pigment itself. instead, it hosts a regulatory enhancer region (rs12913832) that dictates OCA2 transcription levels in melanocytes. This SNP is the single strongest predictor of blue vs brown eyes.
TYR (tyrosinase)
The rate-limiting enzyme for melanin synthesis. variants in TYR modulate how much pigment melanocytes can produce even if OCA2 is active.
TYRP1 (tyrosinase-related protein 1)
Stabilizes tyrosinase and modifies eumelanin vs pheomelanin balance. Mutations here can cause lighter pigment in both iris and skin.
SLC45A2 & SLC24A4
Involved in melanosomal ion exchange and maturation. Variants shift pigmentation efficiency subtly.
IRF4
A transcription factor that modulates TYR expression in a context-dependent way. Weaker IRF4 activity = less melanin production.
Encodes the P protein that regulates pH and ion balance inside melanosomes, indirectly controlling melanin synthesis.
Higher OCA2 expression = more melanin = darker iris. Partial loss-of-function mutations lighten pigmentation.
HERC2
Doesn’t produce pigment itself. instead, it hosts a regulatory enhancer region (rs12913832) that dictates OCA2 transcription levels in melanocytes. This SNP is the single strongest predictor of blue vs brown eyes.
TYR (tyrosinase)
The rate-limiting enzyme for melanin synthesis. variants in TYR modulate how much pigment melanocytes can produce even if OCA2 is active.
TYRP1 (tyrosinase-related protein 1)
Stabilizes tyrosinase and modifies eumelanin vs pheomelanin balance. Mutations here can cause lighter pigment in both iris and skin.
SLC45A2 & SLC24A4
Involved in melanosomal ion exchange and maturation. Variants shift pigmentation efficiency subtly.
IRF4
A transcription factor that modulates TYR expression in a context-dependent way. Weaker IRF4 activity = less melanin production.
In brown-eyed genotypes (GG at rs12913832), the enhancer region actively promotes OCA2 expression in iris melanocytes, leading to high eumelanin deposition.
In blue-eyed genotypes (AA), the enhancer is disrupted, reducing chromatin accessibility for key transcription factors (MITF, LEF1, HLTF), so OCA2 transcription plummets. melanin drops to the point where light scattering dominates, making the iris appear blue.
Heterozygotes (GA) often yield intermediate shades like hazel or green.
In blue-eyed genotypes (AA), the enhancer is disrupted, reducing chromatin accessibility for key transcription factors (MITF, LEF1, HLTF), so OCA2 transcription plummets. melanin drops to the point where light scattering dominates, making the iris appear blue.
Heterozygotes (GA) often yield intermediate shades like hazel or green.
Yes, rs12913832 accounts for ~74–80% of eye color variance in european populations, but the remaining variation is due to polygenic modifiers (TYR, SLC24A4, etc.).
This explains why two people with the same rs12913832 genotype can still have slightly different shades + structural and epigenetic variations influence how much enhancer activity is silenced.
So if you’re designing a genetic intervention, you mainly target rs12913832, but you also accept that background genes set the “baseline” pigment level. If your TYR is hyperactive, you might still have residual pigment even if you downregulate OCA2.
This explains why two people with the same rs12913832 genotype can still have slightly different shades + structural and epigenetic variations influence how much enhancer activity is silenced.
So if you’re designing a genetic intervention, you mainly target rs12913832, but you also accept that background genes set the “baseline” pigment level. If your TYR is hyperactive, you might still have residual pigment even if you downregulate OCA2.
To genetically induce a blue-eyed phenotype in a brown-eyed adult, you mimic the AA genotype at rs12913832 (HERC2) to downregulate OCA2 in iris melanocytes. But complete predictability is impossible without knowing the individual’s full polygenic background.
Sturm et al. (2008): rs12913832 predicts ~68 % of eye‑color variance

[2]
Functional and chromatin‑looping studies: Strongest known predictor (86–99 % in practice)

[3]
And:

[4]
Together these papers confirm that rs12913832 is the dominant determinant (~70–80%), while the remainder of variance comes from other gene interactions (TYR, SLC24A4, TYRP1, IRF4, etc.)
[2]
Functional and chromatin‑looping studies: Strongest known predictor (86–99 % in practice)
[3]
And:

[4]
Together these papers confirm that rs12913832 is the dominant determinant (~70–80%), while the remainder of variance comes from other gene interactions (TYR, SLC24A4, TYRP1, IRF4, etc.)
The main point I'm trying to make here is that rs12913832 in HERC2 is responsible for the vast majority (~68–80 %) of eye color variation between blue and brown. the rest of the variation, especially intermediate or unexpected phenotypes, comes from polygenic modifier effects in TYR, TYRP1, SLC24A4, IRF4, and others.
SECTION III:
The principle of somatic gene editing in the adult iris
If you want to genetically alter your eye color as an adult, you’re dealing with somatic gene editing instead of germline. This distinction is critical, because the iris melanocytes you’re targeting are already fully differentiated and non-dividing, which makes them harder to manipulate than embryonic cells.
Editing adult iris color is feasible only as somatic gene therapy, requiring highly targeted delivery to a stable, non-dividing melanocyte population. Efficiency is inherently low, timelines are long, and the results are really fucking unpredictable.
Germline editing modifies the genome before development, so the embryo grows with the altered genotype. This would produce uniformly blue eyes if you disrupt rs12913832 in the zygote stage. But once you’re an adult, germline edits can’t retroactively change differentiated tissues.
So your only viable route is somatic editing, meaning you alter gene expression in the melanocytes of your iris without affecting the rest of your body.
So your only viable route is somatic editing, meaning you alter gene expression in the melanocytes of your iris without affecting the rest of your body.
The catch is that iris melanocytes are post-mitotic (non-dividing) and relatively quiescent. They don’t constantly renew like skin epidermal cells.
- This means delivery efficiency matters: You need to transduce as many cells as possible in one attempt.
- Pigment remodeling is slow because existing melanin granules persist for months before being degraded and replaced.
So even after a “successful” edit, visible color change will take months to years, depending on how fast your melanocytes remodel their melanosomes.
- This means delivery efficiency matters: You need to transduce as many cells as possible in one attempt.
- Pigment remodeling is slow because existing melanin granules persist for months before being degraded and replaced.
So even after a “successful” edit, visible color change will take months to years, depending on how fast your melanocytes remodel their melanosomes.
Since these cells don’t proliferate, classical editing approaches have low efficiency. Integrating viral vectors (like lentivirus) isn’t ideal because insertional mutagenesis in the eye could cause inflammation or tumors.
The best current candidate is AAV (adeno-associated virus):
- It transfects non-dividing cells
- It’s relatively low immunogenic
- It has precedent in FDA-approved retinal gene therapies (Luxturna for RPE65 blindness)
BUT, AAV can only carry ~4.7kb of genetic payload, forcing you to use compact editing systems like SaCas9 (from Staphylococcus aureus) rather than the larger SpCas9.
The best current candidate is AAV (adeno-associated virus):
- It transfects non-dividing cells
- It’s relatively low immunogenic
- It has precedent in FDA-approved retinal gene therapies (Luxturna for RPE65 blindness)
BUT, AAV can only carry ~4.7kb of genetic payload, forcing you to use compact editing systems like SaCas9 (from Staphylococcus aureus) rather than the larger SpCas9.
- Sequence your genome first to confirm you have the brown-eyed GG genotype at rs12913832.
- Design a CRISPR system (either cutting or CRISPR interference) targeting the rs12913832 enhancer region.
- Package it in an AAV under an iris-melanocyte–specific promoter (e.g. tyrosinase promoter) so you don’t hit skin or retinal cells.
- Inject it directly into the anterior chamber or stroma of the eye for localized delivery.
- Wait months for melanocytes to reduce melanin synthesis and for stromal pigment density to drop.
- Design a CRISPR system (either cutting or CRISPR interference) targeting the rs12913832 enhancer region.
- Package it in an AAV under an iris-melanocyte–specific promoter (e.g. tyrosinase promoter) so you don’t hit skin or retinal cells.
- Inject it directly into the anterior chamber or stroma of the eye for localized delivery.
- Wait months for melanocytes to reduce melanin synthesis and for stromal pigment density to drop.
Because you’re not “removing” existing pigment but rather downregulating new melanin synthesis, old pigment must be degraded naturally.
- Result -> Slow fading, often uneven.
If editing efficiency isn’t uniform, you can get sectoral heterochromia (patchy two-tone iris).
- Result -> Slow fading, often uneven.
If editing efficiency isn’t uniform, you can get sectoral heterochromia (patchy two-tone iris).
Editing adult iris color is feasible only as somatic gene therapy, requiring highly targeted delivery to a stable, non-dividing melanocyte population. Efficiency is inherently low, timelines are long, and the results are really fucking unpredictable.
From “Does melanin turnover occur in the eyes of adult vertebrates?” (1993, Pigment Cell Research):

[5]
This review underlines that even though some melanin production occurs, the actual turnover rates in adult iris tissues remain unknown, implying very slow dynamics.

[5]
This review underlines that even though some melanin production occurs, the actual turnover rates in adult iris tissues remain unknown, implying very slow dynamics.
From “Iris as a recipient tissue for pigment cells: Organized in vivo …” (2008, Developmental Dynamics):

[6]
This indicates that iris melanocytes are far less regenerative than skin melanocytes, reinforcing the fact that editing outcomes unfold over long timescales due to cellular quiescence.

[6]
This indicates that iris melanocytes are far less regenerative than skin melanocytes, reinforcing the fact that editing outcomes unfold over long timescales due to cellular quiescence.
SECTION IV:
Designing the edit - Tools and molecular strategies
If you want to genetically downregulate melanin synthesis in iris melanocytes, you have to choose the right molecular toolset.
The target is clear: disrupt enhancer activity at rs12913832 in HERC2, thereby reducing OCA2 transcription. But how you achieve this matters for both efficiency and safety.
For a realistic 2025-level approach:
CRISPRi with dSaCas9-KRAB in an AAV, under a tyrosinase promoter, targeting rs12913832 is the most controlled, tissue-specific strategy.
Base/prime editing would be ideal in theory, but delivery isn’t mature for adult iris cells yet.
The target is clear: disrupt enhancer activity at rs12913832 in HERC2, thereby reducing OCA2 transcription. But how you achieve this matters for both efficiency and safety.
Classic CRISPR-Cas9 nuclease (DNA cutting)
you design a guide RNA that binds near rs12913832. Cas9 introduces a double-strand break, which is repaired imperfectly (non-homologous end joining), effectively disrupting the enhancer sequence.
Pros: permanent silencing.
Cons: double-strand breaks are risky. Off-target cuts can trigger unwanted mutations, apoptosis, or even immune responses.
CRISPRi (interference)
A dead Cas9 (dCas9) fused to a KRAB repressor domain is guided to rs12913832, epigenetically silencing the enhancer without cutting DNA.
Pros: Safer, reversible if vector expression fades.
Cons: Requires continuous expression of the dCas9-KRAB complex, meaning your AAV must remain active long-term.
CRISPRa (activation)
This is the opposite approach, only useful if you wanted darker eyes. You’d basically fuse dCas9 to VP64 to upregulate OCA2.
Irrelevant here unless you’re going brown-to-darker-brown.
Base editing or prime editing
- Base editors can flip single nucleotides without causing double-strand breaks (e.g., Change rs12913832 GG → AA, mimicking blue-eyed genotype precisely).
- Prime editors allow targeted small edits with a pegRNA template.
Pros: highly precise.
Cons: delivery efficiency is currently poor in ocular tissues; no clinical precedent yet.
So, for 2025 tech: CRISPRi (dCas9-KRAB) is the most logical balance of safety + tissue specificity, unless you’re reckless enough for blunt Cas9 cuts.
you design a guide RNA that binds near rs12913832. Cas9 introduces a double-strand break, which is repaired imperfectly (non-homologous end joining), effectively disrupting the enhancer sequence.
Pros: permanent silencing.
Cons: double-strand breaks are risky. Off-target cuts can trigger unwanted mutations, apoptosis, or even immune responses.
CRISPRi (interference)
A dead Cas9 (dCas9) fused to a KRAB repressor domain is guided to rs12913832, epigenetically silencing the enhancer without cutting DNA.
Pros: Safer, reversible if vector expression fades.
Cons: Requires continuous expression of the dCas9-KRAB complex, meaning your AAV must remain active long-term.
CRISPRa (activation)
This is the opposite approach, only useful if you wanted darker eyes. You’d basically fuse dCas9 to VP64 to upregulate OCA2.
Irrelevant here unless you’re going brown-to-darker-brown.
Base editing or prime editing
- Base editors can flip single nucleotides without causing double-strand breaks (e.g., Change rs12913832 GG → AA, mimicking blue-eyed genotype precisely).
- Prime editors allow targeted small edits with a pegRNA template.
Pros: highly precise.
Cons: delivery efficiency is currently poor in ocular tissues; no clinical precedent yet.
So, for 2025 tech: CRISPRi (dCas9-KRAB) is the most logical balance of safety + tissue specificity, unless you’re reckless enough for blunt Cas9 cuts.
SpCas9 (streptococcus pyogenes)
C.S → ~4.2kb coding sequence
Too large for a single AAV vector once you include promoters and gRNA scaffold
SaCas9 (staphylococcus aureus)
C.S → ~3.2kb coding sequence
- Fits within AAV’s 4.7kb packaging limit
- Similar efficiency with slightly different PAM requirements
For ocular gene therapy using AAV, SaCas9 is preferred.
C.S → ~4.2kb coding sequence
Too large for a single AAV vector once you include promoters and gRNA scaffold
SaCas9 (staphylococcus aureus)
C.S → ~3.2kb coding sequence
- Fits within AAV’s 4.7kb packaging limit
- Similar efficiency with slightly different PAM requirements
For ocular gene therapy using AAV, SaCas9 is preferred.
You don’t want OCA2 expression silenced in retinal pigment epithelium or skin. Why? Well... in short, itcould cause unwanted depigmentation. So you’d use a tissue-specific promoter, like:
Tyosinase (TYR) promoter
Active only in melanogenic cells. Also ensures dCas9 or Cas9 only runs in iris melanocytes.
Tyosinase (TYR) promoter
Active only in melanogenic cells. Also ensures dCas9 or Cas9 only runs in iris melanocytes.
Your AAV would carry:
- SaCas9 (or dSaCas9-KRAB) under a TYR promoter
- A guide RNA targeting rs12913832 enhancer region
Once transduced:
- If cutting, you disrupt enhancer → reduce OCA2 transcription → less eumelanin → scattering dominates → iris appears lighter.
- If CRISPRi, you epigenetically silence enhancer, same outcome but reversible.
- SaCas9 (or dSaCas9-KRAB) under a TYR promoter
- A guide RNA targeting rs12913832 enhancer region
Once transduced:
- If cutting, you disrupt enhancer → reduce OCA2 transcription → less eumelanin → scattering dominates → iris appears lighter.
- If CRISPRi, you epigenetically silence enhancer, same outcome but reversible.
- Cutting = permanent (you can’t “undo” a deletion easily lmfao).
- CRISPRi = reversible (if AAV expression fades or you deliver an activator later).
For cosmetic changes, reversibility is safer, since blue eyes are more photosensitive and prone to UV damage.
- CRISPRi = reversible (if AAV expression fades or you deliver an activator later).
For cosmetic changes, reversibility is safer, since blue eyes are more photosensitive and prone to UV damage.
For a realistic 2025-level approach:
CRISPRi with dSaCas9-KRAB in an AAV, under a tyrosinase promoter, targeting rs12913832 is the most controlled, tissue-specific strategy.
Base/prime editing would be ideal in theory, but delivery isn’t mature for adult iris cells yet.
SECTION V:
Vector delivery and ocular targeting
Designing the molecular edit is one challenge, but getting it into the correct cells without collateral damage is another. The iris sits in an anatomically tricky space, exposed to aqueous humor circulation, yet protected by ocular immune privilege. So vector delivery must REALLY balance precision, efficiency, and safety.
The most plausible delivery method for eye-color gene editing is AAV-based, via anterior chamber or direct stromal injection, using SaCas9/dSaCas9-KRAB under a tyrosinase promoter.
Precision is REALLY vital because off-target tissue transduction risks retinal or systemic depigmentation.
Even with perfect delivery, immune response and uneven pigment remodeling remain major limitations.
- AAV efficiently transduces non-dividing cells, which is essential because iris melanocytes are post-mitotic.
- Ocular immune privilege reduces systemic immune responses, making intraocular delivery safer than systemic injections.
- FDA approval of Luxturna proves AAV works for in vivo ocular therapies.
BUT:
- AAV has a small packaging capacity (~4.7 kb), meaning you must use compact Cas9 variants (like SaCas9) and streamlined promoters.
- Pre-existing immunity to AAV capsids can still trigger local inflammation.
- Ocular immune privilege reduces systemic immune responses, making intraocular delivery safer than systemic injections.
- FDA approval of Luxturna proves AAV works for in vivo ocular therapies.
BUT:
- AAV has a small packaging capacity (~4.7 kb), meaning you must use compact Cas9 variants (like SaCas9) and streamlined promoters.
- Pre-existing immunity to AAV capsids can still trigger local inflammation.
There are three main options for reaching the iris melanocytes:
1. Periocular injection (less invasive)
- Delivers vector into tissue surrounding the eye.
- Low efficiency for iris cells because diffusion barriers limit penetration.
2. Anterior chamber injection (intracameral)
- Directly injects vector into the aqueous humor.
- Closer to iris stroma, but still relies on vector diffusion to melanocytes.
3. Direct stromal injection (most precise, most invasive)
- Uses a fine surgical needle to deposit vector directly into iris stroma.
- Highest transduction efficiency, but carries greater surgical risk (bleeding, scarring, glaucoma).
If you're looking for serious experimental attempts, direct stromal or anterior chamber injection is honestly the most realistic route.
1. Periocular injection (less invasive)
- Delivers vector into tissue surrounding the eye.
- Low efficiency for iris cells because diffusion barriers limit penetration.
2. Anterior chamber injection (intracameral)
- Directly injects vector into the aqueous humor.
- Closer to iris stroma, but still relies on vector diffusion to melanocytes.
3. Direct stromal injection (most precise, most invasive)
- Uses a fine surgical needle to deposit vector directly into iris stroma.
- Highest transduction efficiency, but carries greater surgical risk (bleeding, scarring, glaucoma).
If you're looking for serious experimental attempts, direct stromal or anterior chamber injection is honestly the most realistic route.
You want the edit ONLY in iris melanocytes, not retinal pigment epithelium or skin melanocytes. So:
- A tyrosinase (TYR) promoter is the most common melanocyte-specific promoter, limiting expression to melanin-producing cells.
- Combined with tissue-specific enhancers, this reduces off-target gene silencing elsewhere in the eye.
- A tyrosinase (TYR) promoter is the most common melanocyte-specific promoter, limiting expression to melanin-producing cells.
- Combined with tissue-specific enhancers, this reduces off-target gene silencing elsewhere in the eye.
Even in the immune-privileged eye, AAV delivery can provoke:
- Uveitis (inflammation of the uveal tract).
- Complement activation leading to cell death.
Thus, dosing must be minimal but saturating, and some protocols include topical corticosteroids post-injection to suppress inflammation.
- Uveitis (inflammation of the uveal tract).
- Complement activation leading to cell death.
Thus, dosing must be minimal but saturating, and some protocols include topical corticosteroids post-injection to suppress inflammation.
→ The AAV infects iris melanocytes, introducing Cas9/dCas9 machinery
→ Editing or epigenetic silencing begins
→ Elanin synthesis slows, but existing pigment granules remain until they degrade naturally
→ Color change manifests slowly, possibly unevenly.
→ Editing or epigenetic silencing begins
→ Elanin synthesis slows, but existing pigment granules remain until they degrade naturally
→ Color change manifests slowly, possibly unevenly.
The most plausible delivery method for eye-color gene editing is AAV-based, via anterior chamber or direct stromal injection, using SaCas9/dSaCas9-KRAB under a tyrosinase promoter.
Precision is REALLY vital because off-target tissue transduction risks retinal or systemic depigmentation.
Even with perfect delivery, immune response and uneven pigment remodeling remain major limitations.
From “Safety and Tolerability of AAV in the Anterior Chamber” (IOVS, nonhuman primates):

[11]
This clearly shows that injecting AAV into the anterior chamber can deliver genes to iris-adjacent cells safely and efficiently in a primate model.

[11]
This clearly shows that injecting AAV into the anterior chamber can deliver genes to iris-adjacent cells safely and efficiently in a primate model.
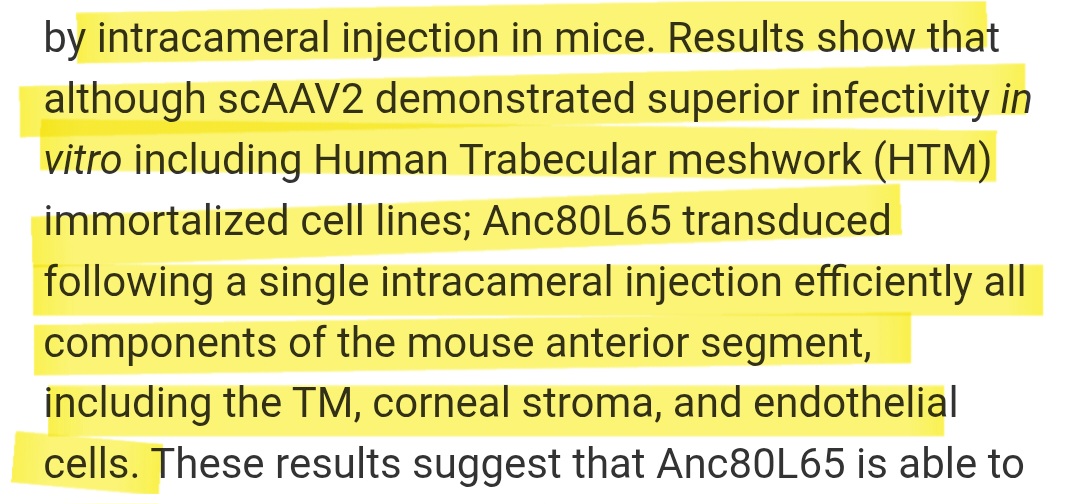
From Wang et al. 2017, PLoS ONE:

[12]
This confirms that specific AAV serotypes can reach and infect anterior tissues relevant to iris melanocytes via eye-syndrome intracameral routes.
[12]
This confirms that specific AAV serotypes can reach and infect anterior tissues relevant to iris melanocytes via eye-syndrome intracameral routes.
SECTION VI.
Risks and physiological trade-offs
We’re not making this long because it doesn’t need to be. These aren’t theoretical risks. They’re obvious biological consequences of messing with pigment cells in your eye. You don’t need 12 studies to know fire burns. same here.
So:
1. Less pigment = more UV → you get light sensitivity
2. Pigment protects from cancer → you lose it, melanoma risk rises
3. Bad gene delivery = patchy edit → you look glitched
4. Immune system notices foreign edits → inflammation, rejection
5. Iris controls light intake → tamper with it, and your vision gets worse
Every single one of these is stuck into what the iris does. If you fuck with the structure, expect structural problems. This is basic biology.
So:
1. Less pigment = more UV → you get light sensitivity
2. Pigment protects from cancer → you lose it, melanoma risk rises
3. Bad gene delivery = patchy edit → you look glitched
4. Immune system notices foreign edits → inflammation, rejection
5. Iris controls light intake → tamper with it, and your vision gets worse
Every single one of these is stuck into what the iris does. If you fuck with the structure, expect structural problems. This is basic biology.
SECTION VII:
Theoretical vs practical timelines of color change
Again, this is one is short, but mainly because the truth is boring.
Despite the hype, the timeline is slow and the results are mid:
- 3–6 months minimum before visible change
- uneven edits = color looks patchy or fake
- low contrast = dull blue or grey, not vivid
- some never see change at all
No study needed to prove biology doesn’t rush. You’re literally editing static pigment cells.
If you're expecting “baby blue in two weeks,” you're a bit retarded.
Despite the hype, the timeline is slow and the results are mid:
- 3–6 months minimum before visible change
- uneven edits = color looks patchy or fake
- low contrast = dull blue or grey, not vivid
- some never see change at all
No study needed to prove biology doesn’t rush. You’re literally editing static pigment cells.
If you're expecting “baby blue in two weeks,” you're a bit retarded.
SECTION VIII:
Future directions - Precision editing, monitoring, and ethical chaos
Even if the current approach is theoretically possible, it’s still primitive compared to where somatic gene editing could head. I'll break this into two future dimensions:
1. Precision editing tools
2. Monitoring and eversibility tech
We’re moving toward an era where precision, reversible, tissue-specific edits will exist. But ethical frameworks lag behind. The same tech that could cure ocular albinism could also mass-produce cosmetic blue eyes. Regulators will likely draw a hard line, but as history shows, if something is technically possible, someone will do it, legally or not.
So:
In the near term, this remains fringe experimental.
In the long term (next 10–20 years), advances in prime editing, tissue-specific delivery, and optogenetic reversibility will make it safer and more controllable.
1. Precision editing tools
2. Monitoring and eversibility tech
Prime editing for rs12913832 genotype switching
Instead of silencing the enhancer, you’d directly convert GG → AA at rs12913832, mimicking the exact blue-eyed genotype.
This would give a clean and predictable blue phenotype rather than a random desaturation.
Current limitation: Prime editing delivery to ocular cells is still inefficient, and no approved ophthalmic prime editor exists.
Epigenetic rewriting without permanent changes
Next-gen CRISPRoff/CRISPRon systems can silence or re-activate genes without cutting DNA, making changes reversible.
Imagine an optogenetic Cas9 tethered to light-sensitive domains, so you could literally “switch your eye color” temporarily with specific light wavelengths.
mRNA + lipid nanoparticle (LNP) delivery
mRNA coding for Cas9 + gRNA can be delivered via LNPs (like COVID vaccines), avoiding viral integration and long-term risks.
Problem: Iris melanocytes are quiescent and hard to transfect with non-viral methods. But advances in tissue-specific LNPs might change this.
Instead of silencing the enhancer, you’d directly convert GG → AA at rs12913832, mimicking the exact blue-eyed genotype.
This would give a clean and predictable blue phenotype rather than a random desaturation.
Current limitation: Prime editing delivery to ocular cells is still inefficient, and no approved ophthalmic prime editor exists.
Epigenetic rewriting without permanent changes
Next-gen CRISPRoff/CRISPRon systems can silence or re-activate genes without cutting DNA, making changes reversible.
Imagine an optogenetic Cas9 tethered to light-sensitive domains, so you could literally “switch your eye color” temporarily with specific light wavelengths.
mRNA + lipid nanoparticle (LNP) delivery
mRNA coding for Cas9 + gRNA can be delivered via LNPs (like COVID vaccines), avoiding viral integration and long-term risks.
Problem: Iris melanocytes are quiescent and hard to transfect with non-viral methods. But advances in tissue-specific LNPs might change this.
Single-cell RNA-seq from aqueous humor sampling
Future clinics could do minimally invasive aspiration of aqueous humor to monitor whether OCA2 expression actually dropped in iris cells.
qPCR and western blot could confirm editing success before pigment change manifests.
Reversible gene control
E.g., dCas9-KRAB expression driven by a doxycycline-inducible promoter, allowing you to silence OCA2 only when you take a specific drug.
Theoretically you could “toggle” between natural and lightened iris states.
Future clinics could do minimally invasive aspiration of aqueous humor to monitor whether OCA2 expression actually dropped in iris cells.
qPCR and western blot could confirm editing success before pigment change manifests.
Reversible gene control
E.g., dCas9-KRAB expression driven by a doxycycline-inducible promoter, allowing you to silence OCA2 only when you take a specific drug.
Theoretically you could “toggle” between natural and lightened iris states.
We’re moving toward an era where precision, reversible, tissue-specific edits will exist. But ethical frameworks lag behind. The same tech that could cure ocular albinism could also mass-produce cosmetic blue eyes. Regulators will likely draw a hard line, but as history shows, if something is technically possible, someone will do it, legally or not.
So:
In the near term, this remains fringe experimental.
In the long term (next 10–20 years), advances in prime editing, tissue-specific delivery, and optogenetic reversibility will make it safer and more controllable.
SECTION IX:
CONCLUSION
What I've just traced is the razor’s edge between what biology allows and what technology can currently force. Canging eye color genetically in an adult is not impossible, but it is:
- Slow as fuck because the biology of iris melanocytes is low-turnover and stubborn.
- Imprecise because delivery vectors don’t saturate uniformly, creating patchy results.
- Dangerous because it trades natural UV protection for aesthetic novelty.
- Ethically gray because it sits outside medicine’s mandate to heal.
Even if you solved delivery with surgical precision, minimized immune reactions, and built in reversibility, you’d still face that: the human body is an interdependent system.
The iris pigment has cascading effects on vision, cancer risk, and ocular health.
- You can silence or edit OCA2/HERC2 in your iris.
- You will likely wait months for uneven, partial results.
- You will risk photophobia, higher melanoma susceptibility, and unknown immune effects.
- You will step into an ethical vacuum where enhancement and medicine blur.
The technology is emerging, but it’s FAR from clean, safe, nor trivial lol.
- Slow as fuck because the biology of iris melanocytes is low-turnover and stubborn.
- Imprecise because delivery vectors don’t saturate uniformly, creating patchy results.
- Dangerous because it trades natural UV protection for aesthetic novelty.
- Ethically gray because it sits outside medicine’s mandate to heal.
Even if you solved delivery with surgical precision, minimized immune reactions, and built in reversibility, you’d still face that: the human body is an interdependent system.
The iris pigment has cascading effects on vision, cancer risk, and ocular health.
- You can silence or edit OCA2/HERC2 in your iris.
- You will likely wait months for uneven, partial results.
- You will risk photophobia, higher melanoma susceptibility, and unknown immune effects.
- You will step into an ethical vacuum where enhancement and medicine blur.
The technology is emerging, but it’s FAR from clean, safe, nor trivial lol.

Attachments
Last edited: