D
Deleted member 15854
Luminary
- Joined
- Oct 26, 2021
- Posts
- 5,920
- Reputation
- 5,174
Chondroinduction in vascular tissue can occur without injury:
Overexpression of transforming growth factor beta1 in arterial endothelium causes hyperplasia, apoptosis, and cartilaginous metaplasia.
"Uninjured rat arteries transduced with an adenoviral vector expressing an active form of transforming growth factor beta1 (TGF-beta1) developed a cellular and matrix-rich neointima, with cartilaginous metaplasia of the vascular media. Explant cultures of transduced arteries showed that secretion of active TGF-beta1 ceased by 4 weeks, the time of maximal intimal thickening. Between 4 and 8 weeks, the cartilaginous metaplasia resolved and the intimal lesions regressed almost completely, in large part because of massive apoptosis. Thus, locally expressed TGF-beta1 promotes intimal growth and appears to cause transdifferentiation of vascular smooth muscle cells into chondrocytes. Moreover, TGF-beta1 withdrawal is associated with regression of vascular lesions."
"Arteries [with positive expression of type II collagen] revealed rounded cells with a high nuclear/cytoplasmic ratio, surrounded by lacunae. A loose extracellular matrix was present, appearing more cartilaginous than vascular, with collagen fibers, abundant proteoglycans, and little elastin"
"The appearance of chondrocytes in the arterial wall was not caused by migration of cells from cartilage "
"TGF-β1 expression leads to increased focal vascular cell proliferation"
Unlike bone, cartilage regeneration remains elusive.
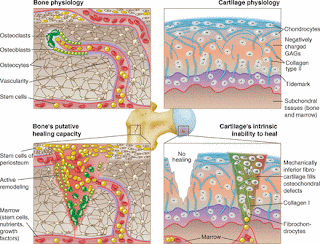
"bone marrow MSCs or resident chondroprogenitor cells [cannot] generate hyaline ECM" Highlighting the importance of hyaluronic acid supplementation.
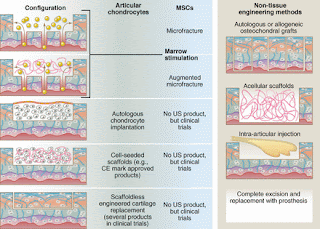
"Microfracture involves subchondral bone penetration to release bone marrow that forms a stem cell–rich clot. "
"Chondro-differentiation of MSCs results in an unnatural differentiation pathway that is unlike either endochondral ossification or permanent cartilage formation in that markers of hyaline cartilage (collagen type II{up} and SOX-9), hypertrophy (collagen type X{up} and MMP13), and bone (osteopontin{up} and bone sialoprotein{up}) are expressed concurrently"
"Cartilage-to-cartilage integration is exceedingly difficult to achieve, because cartilage displays low metabolism and contains dense, anti-adhesive ECM. For example, proteins transcribed from the PRG4 gene, contributors to cartilage’s low friction, and GAGs have been shown to directly inhibit cell adhesion"
Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice.
"Functional suitability and phenotypic stability of ectopic transplants are crucial factors in the clinical application of mesenchymal stem cells (MSCs) for articular cartilage repair, and might require a stringent control of chondrogenic differentiation. This study evaluated whether human bone marrow-derived MSCs adopt natural differentiation stages during induction of chondrogenesis in vitro, and whether they can form ectopic stable cartilage that is resistant to vascular invasion and calcification in vivo.
During in vitro chondrogenesis of MSCs, the expression of 44 cartilage-, stem cell-, and bone-related genes and the deposition of aggrecan and types II and X collagen were determined. Similarly treated, expanded articular chondrocytes served as controls. MSC pellets were allowed to differentiate in chondrogenic medium for 3-7 weeks, after which the chondrocytes were implanted subcutaneously into SCID mice; after 4 weeks in vivo, samples were evaluated by histology.
The 3-stage chondrogenic differentiation cascade initiated in MSCs was primarily characterized by sequential up-regulation of common cartilage genes. Premature induction of hypertrophy-related molecules (type X collagen and matrix metalloproteinase 13) occurred before production of type II collagen and was followed by up-regulation of alkaline phosphatase activity. In contrast, hypertrophy-associated genes were not induced in chondrocyte controls. Whereas control chondrocyte pellets resisted calcification and vascular invasion in vivo, most MSC pellets mineralized, in spite of persisting proteoglycan and type II collagen content.
An unnatural pathway of differentiation to chondrocyte-like cells was induced in MSCs by common in vitro protocols. MSC pellets transplanted to ectopic sites in SCID mice underwent alterations related to endochondral ossification rather than adopting a stable chondrogenic phenotype. Further studies are needed to evaluate whether a more stringent control of MSC differentiation to chondrocytes can be achieved during cartilage repair in a natural joint environment."
"dedifferentiated late-passage chondrocytes lose their ability to form ectopic cartilage and generate only fibrous-like tissue after transplantation"<-Maybe something similar happens physiologically to inhibit new growth plate formation?
In vitro differentiation of MSCs:
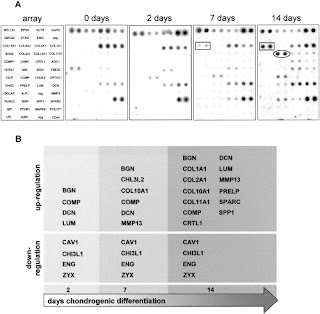
Up in LSJL:
Bgn
Lum
Col10a1
Col11a1
Col2a1
Col1a1
Spp1(as Osteopontin)
The appearance of Col10a1 before Col2a1 is abnormal.
"human adult MSCs derived from bone marrow can be programmed to produce ectopic fibrocartilage rich in proteoglycans and types I, II, and X collagen, and to undergo calcification and vascular invasion consistent with a program related to endochondral ossification. Remarkably, this sequence occurred in the absence of a 3-dimensional carrier and a growth factor depot, and without genetic manipulation of the cells."
The medium to induce chondrogenic differentiation of MSCs included TGFB3 and was pellet culture. The ectopic cultures were implanted on the backs of 8 week old mice. No hydrostatic pressure, tensile strain stimulation, or dynamic compression was used which could help LSJL from more appropriate endochondral ossification cartilage than present in this study.
Sequence of development of innately regenerated growth-plate cartilage in the hindlimb of the neonatal rat
"the neonatal rat can regenerate the distal femoral growth-plate. The age of the rodent and level and angle of amputation as significant modifiers of the regeneration process. Examination of these issues constitutes the objective of the present report. Fifty-four male, outbred albino rats sustained low femoral (48 rats) or midtibiofibular (six rats) hind-limb amputations when ten to eleven days old. They were killed after 0, 1, 2, 4, 7, 14, 22 or 29 postoperative days; and their amputation stumps were sectioned longitudinally. Twenty-four hr after amputation, the distal femoral periosteum was thickened and metachromatic regions were observed forming within it. Intraperiosteal cartilage was observed by the end of the second postoperative day in four of six limb stumps and, during the following week, expanded considerably in volume. Regenerated growth-plate cell architecture was recognized within the enlarging cartilage mass by the end of the second week; and, by the end of the fourth postoperative week, the regenerating growth-plate region had achieved considerable architectural maturity."
Rats lose the ability to regenerate growth plates after 5 months of age and are only able to regenerate growth plates in the lower but not upper extremities.
"Among rats killed two or four days after surgery, clots were present at the amputation surface and had begun to infiltrate between the nonskeleta1 tissues of the limb stumps, appearing in intermuscular spaces and along fascia1 planes."
"[48 hours post operation] Four of six femurs displayed small, irregular bodies of hyaline cartilage forming within the distal periosteum"
"[96 hours post operation] All seven amputation stumps displayed expanding cartilage bodies applied to their femoral termini. This cartilage formed a cuff surrounding the skeletal terminus, and within it ossification was proceeding as indicated by the appearance of delicate trabeculae of newly formed endochondral bone"
"[3 weeks post operation] new bone extended beyond the original amputation plane for a considerable
length, but in no specimen was evidence of distinct growth-plate regeneration observed."
Signs of a new growth plate appeared 4 weeks post operation. In this type of growth plate regeneration the new growth plates appear de novo and not from tissues of the same type.
"the regenerated physis is formed via proliferation of periosteal cells which rapidly transform into chondrocytes"
"Unlike the amphibian limb, the regeneration of the growth-plate in the mammalian hindlimb occurs even after closure of the skin with structures"
Ectopic osteogenesis and chondrogenesis of bone marrow stromal stem cells in alginate system
"The present study sought to determine the ectopic osteogenic and chondrogenic ability of BMSSCs in combination with a scaffolding material made from alginate gel. After isolation from the bone marrow of BALB/C [8 week old male] mice, BMSSCs were expanded in vitro and induced to chondrogenesis or osteogenesis for 14 days, respectively. Subsequently, these induced cells were seeded into alginate gel, and the constructs implanted into BALB/C nude mice subcutaneously for up to 8 weeks. In the histological analysis, the transmission electron microscopy of the retrieved specimens at various intervals showed obvious trends of ectopic cartilage or bone formation along with the alteration of the cellular phenotype. Simultaneously, the results of the immunohistochemical staining and RT-PCR both confirmed the expression of specific extracellular matrix (ECM) markers for cartilaginous tissue, such as collagen type II (Col-II), SOX9, and aggrecan, or alternatively, markers for osteoid tissue, such as osteopontin (OPN), osteocalcin (OCN), and collagen type I (Col-I). During subcutaneous implantation, the elevating production of ECM and the initiation of the characteristic structure were closely correlated with the increase of time. In contrast, there was an apparent degradation and resorption of the scaffolding material in blank controls, but with no newly formed tissues. Finally, the constructs that were made of non-induced BMSSCs nearly disappeared during the 8 weeks after implantation. Therefore, it is suggested that alginate gel, which is combined with BMSSCs undergoing differentiation into skeletal lineages, may represent a useful strategy for the clinical reconstruction of bone and cartilage defects."
"After placed into the chondrogenic medium, BMSSCs were modulated from an elongated fibroblastic appearance to a smaller polygonal or round shape. The immunocytostaining of Col-II was a strong positive in 80% of the cells. When the chondrogenic induced BMSSCs were seeded into alginate, the scanning electronic microscopy results show the cells to be well attached to the scaffolds. Subsequently, the BMSSCs/alginate was implanted into 6-week-old female BALB/C nude micesubcutaneously."
"As shown in the immunostaining, Col-II was positive at 8 weeks after implantation"
"Col-II, aggrecan, and SOX9 were observed in differentiated monolayer cells and engineered constructs that were not observed in the controls "
In the osteogenic medium "With HE staining, the experimental implants showed surrounding fibrous tissue, and a mild infiltration of inflammatory cells and fibroblasts at 4 weeks. The resorption of alginate and endochondral ossification appeared to some extent, but there was no obvious initiation of bone formation at this point"<-indicating that chondroinduction can occur in an osteogenic medium too.
" in the osseous constructs, the phenomenon of endochondral ossification occurred at 4 weeks, and the structure of bone trabecula containing a large number of cells and collagenous ECM formed in another 4 weeks. "
"Although differentiated BMSSCs were not actual chondrocytes or osteoblasts, their chondrogenic and osteogenic nature was confirmed with not only morphological features but also immunohistochemical staining of the marker proteins in cartilage and bone."<-maybe mechanical stimulation could cement their phenotype.
"In the neo-cartilage, the intensity of mRNA encoding aggrecan, SOX9, and Col-II maintained stably at high levels as shown in the RT-PCR results. Correspondingly, there was a significant accumulation of ECM components in the pericellular matrix with increasing time. The same trend of Col-I, OCN, and OPN production was also found in the new bone, which promoted the confluence of ECM and the development of a bony structure."
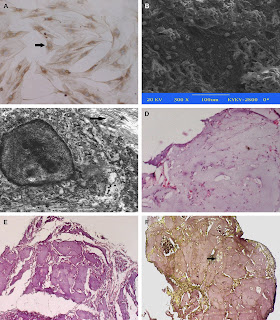
"Morphological and gross observation of cartilage tissues. Two weeks after chondrogenic induction, the BMSSCs indicated a smaller polygonal or round shape as is in chondrocytes. The immunocytostaining of Col-II was a strong positive in 80% of the cells, the arrow points to the positive cell (A). When the chondrogenic induced BMSSCs were seeded into alginate, the scanning electronic microscopy results show the cells to be well attached with the scaffolds (B). The engineered chondroid constructs formed white spheroid aggregates and were capsuled by connective tissues. The ultramicro cellular observation indicated the specific markers of cartilage formation, such as the presence of abundant ribosome and rough endoplasmic reticulum, and pericellular collagen fibrils and proteoglycan granules at 4 weeks. The arrow points to the collagen fibers (C). HE staining indicated the initial cartilage formation after 4 weeks of implantation (D) and neo-cartilage becoming confluent and mature at 8 weeks (E). The 8 weeks constructs positively stained with the antibodies against Col-II proteins, the arrow points to the lacunae (F)."
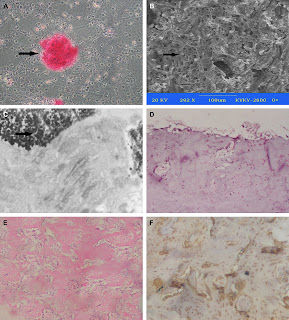
"Morphological and gross observation of osteoid tissues. After culture in an osteogenic medium for two weeks, the BMSSCs became a cuboidal shape and were surrounded with an abundant matrix, when confluent, the monolayer cells aggregated to form nodules with differentiated cells trapped in an abundant matrix, which was positive in alizarin red S. The arrow points to the calcified nodule (A). The retrieved osteoid constructs exhibited white, rigid constitution and the alginate content decreased stepwise. When the osteogenic induced BMSSCs were seeded into alginate, the scanning electronic microscopy results show the cells to be well attached with the scaffolds, the arrow points to the cells attached on the scaffold (B). The transmission electron microscopy indicated an abundance of ribosome and rough endoplasmic reticulum, and pericellular paralleling collagen fibrils at 4 weeks, and there were many vacuoles in cytoplasm and calcification in some areas, the arrow points to the calcification area (C). HE staining indicated the resorption of alginate and endochondral ossification in constructs after 4 weeks of implantation with the surrounding fibrous tissue and a mild infiltration of inflammatory cells and fibroblasts (D). After 8 weeks, remnants of a little alginate were distributed in the partially mineralized ECM and new bone formation appeared in a closely knitted arrangement throughout the implanted constructs (E). In an OCN immunohistochemical assay, which is a marker of osteoblasts, the extensively regenerated bone was confirmed and the structure of bone trabecula contained an abundance of cells and collagenous ECM (F). There was no detectable signal of bone formation in the blank controls."
So ectopic chondrossification is theoretically possible.
Transcriptional mechanisms in osteoblast differentiation and bone formation
"Osx-null cells acquire a chondrocyte phenotype implies that Osx is a negative regulator of Sox9 and of the chondrocyte phenotype."
" if Sox9 is inactivated after the establishment of mesenchymal condensations, Runx2 expression and osteoblast differentiation takes place."
Perichondrium phenotype and border function are regulated by Ext1 and heparan sulfate in developing long bones: A mechanism likely deranged in Hereditary Multiple Exostoses
"During limb skeletogenesis the cartilaginous long bone anlagen and their growth plates become delimited by perichondrium with which they interact functionally. Despite being so intimately associated with cartilage, perichondrium acquires and maintains its distinct phenotype and exerts its border function. Because perichondrium becomes deranged and interrupted by cartilaginous outgrowths in Hereditary Multiple Exostoses (HME), a pediatric disorder caused by EXT mutations and consequent heparan sulfate (HS) deficiency, we asked whether EXT genes and HS normally have roles in establishing its phenotype and function. Indeed, conditional Ext1 ablation in perichondrium and lateral chondrocytes flanking the epiphyseal region of mouse embryo long bone anlagen –a region encompassing the groove of Ranvier– caused ectopic cartilage formation. A similar response was observed when HS function was disrupted in long bone anlagen explants by genetic, pharmacological or enzymatic means, a response preceded by ectopic BMP signaling within perichondrium. These treatments also triggered excess chondrogenesis and cartilage nodule formation and overexpression of chondrogenic and matrix genes in limb bud mesenchymal cells in micromass culture. Interestingly, the treatments disrupted the peripheral definition and border of the cartilage nodules in such a way that many nodules overgrew and fused with each other into large amorphous cartilaginous masses. Interference with HS function reduced the physical association and interactions of BMP2 with HS and increased the cell responsiveness to endogenous and exogenous BMP proteins. In sum, Ext genes and HS are needed to establish and maintain perichondrium's phenotype and border function, restrain pro-chondrogenic signaling proteins including BMPs, and restrict chondrogenesis."
So is Ext1 removal can induce ectopic cartilage formation can it also increase height?
"HME is characterized by cartilaginous and bony outgrowths (exostoses) that form next to, but never within, the growth plates." Apparently HME results in short rather than tall stature(most of the time).
"when we compared the frequency of ectopic cartilage formation with respect to the long bone longitudinal axis, it was clear that the incidence was higher in the epiphyseal than diaphyseal region by a ratio of about 4 to 1"
"the epiphysis needs to enlarge and expand laterally by appositional growth and achieve a much larger diameter compared to the diaphysis"
"it is conceivable and possible that the ectopic cartilage formation occurring in Ext1-deficient long bone anlagen in vivo or explant culture is an amplification of that natural process propelled by ectopic pro-chondrogenic signaling activity, increased availability of “free” chondrogenic factors, and precocious recruitment of progenitor cells into the chondrogenic lineage."
Specific inductive potential of a novel nanocomposite biomimetic biomaterial for osteochondral tissue regeneration.
"Osteochondral lesions require treatment to restore the biology and functionality of the joint. A novel nanostructured biomimetic gradient scaffold was developed to mimic the biochemical and biophysical properties of the different layers of native osteochondral structure. The scaffold presents important physicochemical characteristics and can support the growth and differentiation of mesenchymal stromal cells (h-MSCs), which adhere and penetrate into the cartilaginous and bony layers. H-MSCs grown in chondrogenic or osteogenic medium decreased their proliferation during days 14-52 on both scaffold layers and in medium without inducing factors used as controls. Both chondrogenic and osteogenic differentiation of h-MSCs occurred from day 28 and were increased on day 52, but not in the control medium. Safranin O staining and collagen type II and proteoglycans immunostaining confirmed that chondrogenic differentiation was specifically induced only in the cartilaginous layer{finding why chondrogenic differentiation only occurred in the cartilaginous layer could be key to finding methods to induce ectopic chondrogenesis}. Conversely, von Kossa staining, osteocalcin and osteopontin immunostaining confirmed that osteogenic differentiation occurred on both layers."
Control chondrogenic medium and chondrogenic medium were the same except TGF-B1 was added to the chondrogenic medium.
So TGFB-1 is key for chondrocyte differentiation.
Cartilage formation in growth plate and arteries: from physiology to pathology
"vascular smooth muscle cells (VSMCs) undergo chondrogenic commitment eventually leading to vascular calcification, by mechanisms similar to those governing ossification in the cartilage growth plate."
"VSMCs express Sox9 and type II and IX collagen"
An artery carries blood away from the blood into the body. There are arteries in the bone. If ectopic growth plates can be induced in these arteries than it could be possible to form new growth plates.
Posted by Tyler Christopher Davis at 3:53 PM 5 comments
Labels: chondro-ossification
Tuesday, December 18, 2012
More LSJL studies on longitudinal growth!
Since I have bumped this several times I have highlighted the important new relevant information with (*NEW*). You can control-F to find it if you have not read this post I recommend you do because it has a lot of promising LSJL information. For the first time, using the same 16 week old C57/BL/6 rats a length increase was given for the LSJL study but not in the axial loading study. Thus providing further evidence that LSJL is a novel height increasing loading mechanism.
Here's a study that shows that LSJL increases height in adult rats! This is evidence that LSJL could possibly induce adult height growth.
Knee loading promotes longitudinal bone growth in both young and adult mice
"21 young (8 weeks of age) and 15 adult (16 weeks of age) C57/BL/6 female mice were used{rodents retain a cartilagenous template post growth plate cessation however if LSJL still involves differentiation of stem cells into chondrocytes then it can still work in human adult mice it'll just be harder as mice already have a cartilagenous matrix to work with. Also, adult female mice tend to stop growing at 6 months as opposed to the four month old mice used in the study}. The left hindlimb was given 5-min loading bouts (0.5 N at 5 Hz) for 10 days (young mice) and 20 days (adult mice), while the right hindlimb was used as sham loading control. Mice were sacrificed 2 weeks after the last loading
Knee loading lengthened tibiae in both young and adult mice. Compared to sham loading control, the longitudinal tibia length was elevated by 2.3% from 16.68 ± 0.06 mm (control) to 17.06 ± 0.05 mm (loading) in young mice and 1.5% from 17.82 ± 0.04 mm (control) to 18.09 ± 0.03 mm (loading) in adult mice{so the growth plates were not senescent in adult mice which means that it is not proof that LSJL induces chondrogenic differentiation}. The tibia weight was increased by 10.4% in young mice and by 6.0% in adult mice. Loads elevated BMD and BMC. In young mice, for instance, BMD was increased from 0.0409 ± 0.0003 g/cm2 (control) to 0.0427 ± 0.0003 g/cm2 (loading) and BMC was elevated from 0.0136 ± 0.0002 g (control) to 0.0145 ± 0.0002 g (loading).
Knee loading enhances longitudinal bone growth in both young and adult mice [with stronger effects in young mice]. Joint loading may have a potential usage in development of load-driven therapies for limb length discrepancy and short stature."
The adjunct LSJL scientist Stuart J. Warden did an axial loading study also on 16 week old female C57/BL/6:
Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model.
"Adult (16weeks old) female C57BL/6 mice were randomly divided into three load magnitude groups (5, 7 and 9N), and had their right tibia axially loaded using a continuous 2-Hz haversine waveform for 360cycles/day, 3days/week for 4 consecutive weeks." The Newtons used was lower in LSJL than Warden's study. The frequency was 5Hz in LSJL versus 2Hz here. 20 days were used in the LSJL study versus 28 days here which means that the axial loading rats had more time to grow.
"Bone strain on the medial surface of the midshaft tibia demonstrated a linear increase in response to incremental externally applied loads (R2 = 0.99), with 9 N inducing a tensile strain of 1,833 με "
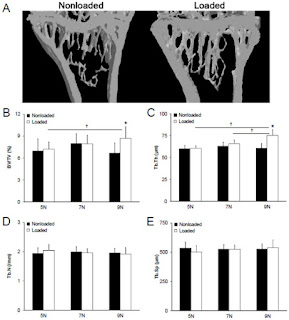
"The ultimate finding of the study was that a load of 9 N (engendering a tensile strain of 1,833 με on medial surface of the midshaft tibia) was able to simultaneously induce lamellar cortical and trabecular bone adaptation when using the mouse tibial axial compression loading model in 16 week old female C57BL/6 mice."
Since the scientists took measurements related to "Effect of loading and load magnitude on percent difference in polar moment of inertia (IP) between loaded and nonloaded tibiae at 1 mm increments along the bone length." They would have had to measure the bone length.
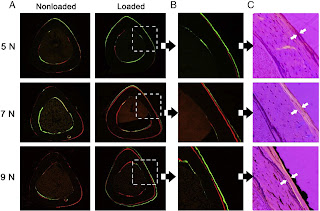
These images serve to contrast against the images in the LSJL study here. The female mice in that study were 14 weeks old. In the LSJL study you can clearly see the degradation of bone in the non-microfracture mice. Thus, in contrast to axial loading, LSJL induces bone degradation which may allow for new growth plate formation.
Absence of proof is not proof of absence so it's possible that there was a length increase in the mice but it was not mentioned so I will be investigating. If I don't get an answer due perhaps to my reputation I will need your help to find out what the length change is. Specifics would be very helpful even if the results are not statistically significant to compare to the LSJL study.
(*NEW*)
Here's a patent related to LSJL:
"The methods and apparatus provide a mechanical load applied to the epiphysis of a bone associated with a joint{just like we are trying to do with LSJL}. The magnitude of the mechanical load is oscillated and is applied periodically for a short durations of time{this oscillation could be important to LSJL as both fluid flow and compression are important for inducing chondrogenesis, the current method of LSJL may not induce enough fluid flow}. The applied mechanical force induces formation of trabecular and cortical bones. Embodiments of the apparatus include passively and actively actuated bands which are positioned around at least a portion of a joint and provide mechanical loading on the joint{so loading the joint capsule is important for LSJL in addition to loading the bone epiphysis}, for example, on the epiphysis of a bone and oriented traverse to the longitudinal axis of the bone{so essentially lateral loading}."<-We are not really oscillating the loads but I suppose we could with the clamp. Rotate the clamp tighter than loosen a little, then a little tighter than before and so on...
Some difficulties for an LSJL like device:
"Generating strain in the diaphysis in limb bones requires a loading device that is large enough to span the entire length of a long bone, for example, a femur or tibia{this device however was designed to stimulate bone formation in the entire bone whereas we are only looking for chondrogenic differentiation in the epiphysis}. Accordingly, the portability and ease of use of such devices are detrimentally affected. In addition, it may be difficult to determine the appropriate loading magnitude for particular individuals; therefore, operation of such a device may cause unwanted bone fractures{<-no reported bone fractures have been reported with LSJL however}. Furthermore, enhancing bone formation by generating a strain in the diaphysis may require that all bones be treated independently{bones have different shapes and differences in other bones present at joints}. Moreover, the use of such devices can require dedicated exercise time which may not be easily incorporated into daily activities[this is an issue people have been having with LSJL]."
Here's some methods described to perform LSJL. They are not specifically described to generate height increase but they are all designed to increase intramedullary pressure and fluid flow which is our goal as to generate chondrogenic differentiation:
"The method of enhancing bone formation in a mammal can include applying a mechanical load to a joint of the mammal so that viscoelastic joint tissues of the joint are deformed{this is what we do with the clamp or dumbell}. In one embodiment the method includes oscillating the amplitude of the mechanical load, for example at about 2 Hz, so that fluid flow is generated in the bones forming the joint. The fluid flow can be interstitial cellular fluid flow and include the diaphysis of a bone. The method can also include varying the amplitude of the mechanical load to generate a streaming potential measured on the periosteal surface of the diaphysis of the bones forming the joint, for example so that the peak-to-peak amplitude of the mechanical load is about 0.5 N. The joint can be, but is not limited to, one of the knee, ankle, hip, shoulder, elbow, and wrist, and the mechanical load in one embodiment is applied laterally to the joint to cause viscoelastic deformation of joint tissues. The method can also include applying a circumferential band around at least a portion of the joint and exercising the joint to vary the load applied to the joint by the band{basically like putting a ring on the finger or something like a ring on your leg. I think it's basically describing tying off the joint and then exercising to induce fluid flow basically like the cuff used to measure blood pressure only over the epiphysis}. The method can also include positioning a wall of a bladder in contact with the joint and advancing a fluid into the bladder{a bladder is a hydration system like the platypus, the wall would be one side of the bag that holds the fluid, so you would put the side of the bladder onto the joint and then put fluid into the bladder(something dense would work best)}. Alternatively, applying the mechanical load to the joint can include positioning a band around at least a portion of the joint and tightening the band.
In another embodiment the method of enhancing bone formation in a mammal having a joint connecting a first bone and a second bone includes applying a mechanical load to the epiphysis of at least one of the first bone and the second bone. The magnitude of the mechanical load can be oscillated and the mechanical load can be applied laterally to the joint such that fluid flow is generated in at least one of the first bone and the second bone. The orientation of the mechanical load can be in a direction that is about transverse to the longitudinal axis of the bone and can be applied to the joint tissue connecting the first bone and the second bone{so you would stack your joints one on top of each other and then load the top joint with a dumbell and hope that you get both joints}."
The hydration system would be interesting to try. A platypus hydration system is available for about 30$. We'd have to find the best liquid to put in the platypus. We'd want the most dense liquid but that's still save and affordable(not something like mercury). For the circumferential band, you could tie off two exercise bands at the end of two joints and then go running.
"In yet another embodiment, the apparatus for enhancing bone formation includes a band configured to fit around at least a portion of a joint of a mammal, a bladder coupled to the band, and a pump in fluid communication with the bladder and operable to advance a fluid, for example water or air, into the bladder. The pump can have a controller for oscillating the volume of fluid in the bladder. The band can form a loop having an adjustable circumference. The band can also be a belt that defines a loop having a circumference configurable to fit around a joint and an electric motor operatively coupled to the belt and capable of varying the circumference of the loop. The band can alternatively include an electro-chemical material, for example, a polymer that undergoes dimensional changes upon exposure to an electric field, such as polypyrrole or polythiophene. Oscillation of the electric field can be used to change the circumference of the loop, thereby varying the mechanical load on the joint.
In another exemplary embodiment, the apparatus for enhancing bone formation includes a band configured to be positioned around a joint and an element coupled to that band that is configured to apply lateral pressure to the joint. The band can include an elastic wrap. The element can also include a pad, for example a fluid filled bladder. In one embodiment two pads are positioned on opposite sides of the joint."
"One exemplary method of the present invention uses exemplary apparatus to provide brief periods of periodic mechanical loads for example for three minutes per day. Additionally, mechanical loads may be oscillated in magnitude, for example sinusoidally between 2 Hz and 30 Hz with a peak to peak load sufficient to induce bone formation enhancing interstitial fluid flow, for example, about 100 N"
"That may be driven by air pressure approximately 40 kPa (5.8 psi) was needed to provide 0.5 N to murine elbows in the second exemplary study discussed below. Assuming that 100 N is used to press a lateral wall of a human knee joint, 51 kPa (7.4 psi) is required for a 50 mm in diameter bladder"<-So we can figure out that 40 kPa produces 0.5N is for mice. So humans need 11more kPa of pressure to generate 0.5N of force given a 50mm in diameter bladder. So for humans 51 kPa per 0.5N of force given a 50mm bladder.
"a micro air pump driven at 6-24 NDC with 180 g weight (available from Sensidyne, of Clearwater, FL)."
"ctuators can be formed in any shape, for example, belts, and it can easily generate strain of 10 % and stress above 20 MPa with a small amount of electricity. For example, a conductive polypyrrole polymer (available from EAMEX Co., of Osaka, Japan) with a cross-sectional area of 50 mm (width) x 3 mm (thickness) can generate 189 Ν"
We can combine clamping and a bladder hydration system. We could clamp the sides of the bone while putting the platypus hydration system on top using just water at this time. Anyone have ideas for substances more dense than water?
Elbow loading promotes longitudinal bone growth of the ulna and the humerus.
"Mechanical stimulation plays a critical role in bone development and growth. In view of recently recognized anabolic responses promoted by a joint-loading modality, we examined the effects of elbow loading on longitudinal growth of the ulna and the humerus. Using a custom-made piezoelectric loader, the left elbow of growing C57/BL/6 female mice was given daily 5-min bouts of dynamic loading for 10 days. The right forelimbs of those mice served as contralateral controls, and the limbs of non-treated mice were used as age-matched controls. The effects of elbow loading were evaluated through measurement of bone length, weight, bone mineral density (BMD), and bone mineral content (BMC), as well as mRNA expression levels of load-sensitive transcription factors such as c-fos, egr1, and atf3. The results revealed that the humerus was elongated by 1.2% compared to the contralateral and age-matched controls, while the ulna had become longer than the contralateral control (1.7%) and the age-match control (3.4%)[We can't be sure if these actually increased final height or merely accelerated height growth until we actually identify how lateral joint loading increases growth]. Bone lengthening was associated with increases in bone weight, BMD and BMC. Furthermore, the mRNA levels of the selected transcription factors were elevated in the loaded ulna and humerus. Interestingly, the increase was observed not only at the elbow but also at the wrist and shoulder in the loaded limb{loading at one area likely changes hydrostatic pressure throughout the bone and the periosteum of one bone is connected to another}."
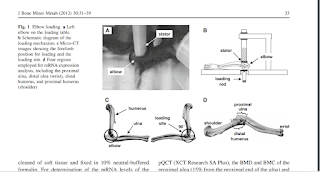
"Loads were 0.5 N at 5 Hz and given for 5 min per day for 10 days . The lateral
and the medial sides of the ulna and humerus were in contact with the loading rod and the stator, respectively. We chose a forelimb configuration that made the right angle (90) between the ulna and the humerus, since in this position the forelimb was relaxed and stably immobilized. To position the elbow properly for loading, the lower end of the loading rod and the upper end of the
supporter (nylon screw) were designed to form a pair of semi-spherical cups. The olecranon process and coronoid process of the ulna together with the ulnar tuberosity, and medial and lateral epicondyles of the humerus were confined in the cups. The tip of the loader had a contact surface of 3 mm in diameter. To avoid a local stress concentration between the elbow and the loader, both the loading surface
and supporter were covered with silicon rubber. The right elbow was used as a sham loading control (contralateral control), which was placed under the loader with no dynamic loading. In the age-matched control group, the same procedure was applied without application of lateral
loads."
In contrast to the other LSJL lengthening study, the contralateral humerus did not increase in length. However, the contralateral ulna did increase in length. If this was a periosteal signaling or a fluid flow related reasons than you'd expect the humerus to increase in length but not the ulna as the ulna's are closer to each other than the humerus'. The length increase was larger in the ulna than humerus so there may be a threshold of adaptation that occurs before the other limb begins to lengthen to compensate. Messenger RNA expression was much higher in the ula than humerus which could be another reason.
"During the loading experiment no bruising or other damage was detected at the loading site"
"We did not observe any load-driven damage in the subchondral bone and the articular cartilage in the humerus and ulna."
A statement from Hiroki Yokota: "We did not conduct histological analysis. I do not know likelihood but it is possible that microdamages are induced in the subchondral bone."
"the load-drive fold change was 4.5 (c-fos), 4.0 (egr-1), and 6.0 (atf3) in the proximal ulna, and 2.9 (c-fos), 3.1 (egr-1), and 3.8 (atf3) in the distal humerus."
"elbow loading (1,500 daily cycles for 10 days) stimulates longitudinal bone growth in the humerus and the ulna. Compared to the contralateral controls, longitudinal length was increased on average by 1.2% (humerus) and 1.7% (ulna). Compared to the age-matched controls, these increases were 1.2% (humerus) and 3.4% (ulna)."
"In response to lateral loads applied to the knee ex vivo, the maximum strain at the loading site was in the order of a few millistrains[10^-3] and the strain at the midshaft cortical bone was in the order of 10 microstrains[10^-6]{So the strain is much higher on the loading site than in the middle of the bone which is good because the loading site is near the epiphysis which means that LSJL may induce more than the 1500 microstrain required to induce bone adaptation}. The longitudinal strain along the bone length was positive, indicating that tensile force acts in the growth plate. As a biophysical mechanism for induction of bone formation with joint loading, it is proposed that alterations in intramedullary pressure are induced and interstitial molecular transport is activated by a dynamic pressure gradient"
According to A comprehensive characterization study of human bone marrow mscs with an emphasis on molecular and ultrastructural properties, c-Fos is a neurogenic marker. Fos- and Jun-related transcription factors are involved in the signal transduction pathway of mechanical loading in condylar chondrocytes states that overexpression of c-Fos inhibits chondrogenic differentiation. LSJL did upregulate C-Fos along with ATF3 and egr1. According to Gene expression profiling of primary human articular chondrocytes in high-density micromasses reveals patterns of recovery, maintenance, re- and dedifferentiation, egr1 is associated with the chondrocyte differentiation process.
According to Egr-1 mediates transcriptional repression of COL2A1 promoter activity by interleukin-1β, egr-1 is an inhibitor of COL2A1 via interleukin-1Beta.
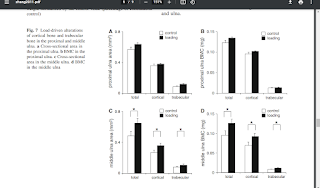
"In the hypertrophic zone of the growth plate in the proximal tibia, the number of chondrocytes and their cellular height were elevated{increase in number of chondrocytes indicates possibility of new stem cells differentiating into chondrocytes thus bypassing proliferative capacity, also increase in cellular height means more height growth per chondrocyte thus also likely leading to a resultant final adult height rather than more acceleration}. Thus, joint loading is potentially useful to lengthen long bones in the hindlimb and the growth plate at the loading site exhibits morphological alterations; however, no studies have been conducted for the forelimb."
"mice were mask-anesthetized using 1.5% isoflurane and received loads to the left elbow in the lateral-medial direction with the custom-made piezoelectric mechanical loader"<-the mice were anesthetized meaning no muscle contraction but that doesn't mean that muscle contraction can't increase hydrostatic pressure.
"Loads were 0.5 N at 5 Hz and given for 5 min per day for 10 days"<-5 bouts of 1/2 the force of gravity per second. Hard to convert this to our purposes but the load seems pretty low. I am now loading at 100 seconds. Perhaps lower loading is needed for LSJL and longer duration. This study uses electricity but a pizeoelectric current is generated by the bone in response to deformation due to loading thus the same effect is achieved due to loading.
"To avoid a local stress concentration between the elbow and the loader, both the loading surface and supporter were covered with silicon rubber"<-thus supporting the usage of socks and clothing to eliminate some of the irritation of LSJL.
"The longitudinal strain along the bone length was positive, indicating that tensile force acts in the growth plate[so the growth plate was stretched but previous growth plate stretching methods have had a lack of success]. As a biophysical mechanism for induction of bone formation with joint loading, it is proposed that alterations in intramedullary pressure[Hydrostatic pressure is likely a subset of intramedullary pressure thus inducing chondrocyte differentiation] are induced and interstitial molecular transport is activated by a dynamic pressure gradient"
"dynamic tensile stress along the length of the ulna and the humerus as well as alterations in intramedullary pressure [may contribute to the bone lengthening]. We observed differential sensitivity to elbow loading in the humerus and ulna. A potential factor for the observed difference may originate from anatomical dimensions and geometries, micrometric and nanometric structures in the lacunocanalicular network, and populations of osteocytes and osteoblasts. In the present study, the loading frequency is 5 Hz. Depending on loading frequencies, it might be possible that the humerus becomes more responsive to elbow loading that the ulna."
"Load-induced longitudinal growth suppression has been reported to be proportional to load magnitude in the growing rat ulna[thus perhaps growth stimulation by lateral joint loading may be proportional to load magnitude as well], but it is not known whether the change in growth rate is also proportional to the number of loading bouts per day or the number of loading days. It is conceivable that the rate of bone lengthening is dependent on various loading conditions such as load magnitude, frequency in Hz, number of bouts per day, and loading duration in days as well as animal age. In summary, the current study demonstrates that elbow loading is an effective means to promote longitudinal bone growth in the mouse humerus and ulna. Joint loading may therefore be potentially useful for the development of load-driven therapies for limb length discrepancy and short stature."
According to Artificial ants deposit pheromone to search for regulatory DNA elements., egr-1 has a strong involvement in chondrogenesis. Other genes of note are E47, CREB, AP-1, AP-2 and Erg-1.
There's also been a review study recently that Hiroki Yokota is a part of that mentions LSJL and may provide some insight:
Mechanical intervention for maintenance of cartilage and bone.
"Moderate loads to the synovial joint suppress the expression levels of matrix metallproteinases (MMPs), while loads above a threshold tend to increase their destructive activities{although some catabolic effects of MMPs may be good for height growth, MMPs may degrade bone allowing for cartilage growth}."
"Moderate shear stress(2–5 dyn/cm2) reduced MMP expression levels, while high shear stress (10–20 dyn/cm2) increased them. Similarly, moderate hydrostatic pressure (1–5 MPa) suppressed MMP-1 expression, while higher loads (10 MPa) elevated it."<-you're likely not to get above 5MPa of hydrostatic pressure even with very large loads so that is not really an issue with LSJL.
"The required magnitude of loads for joint loading is in general smaller than that for axial loading (e.g. 0.5 N for elbow loading and 2–3 N for ulna axial loading in mice). Bone is less stiff in a lateral direction than an axial direction."<-Note that more than 0.5N(100N is mentioned) is likely required for humans. 0.5N is what was used in the mouse arm lengthening study.
"It has been proposed that joint loading periodically alters the pressure in the medullary cavity and activates molecular transport in a lacunocanalicular network in cortical bone."<-It is our hypothesis that this increase in pressure in the medullary cavity induces chondrogenic differentiation. The medullary cavity is continuous into the spaces of the spongy bone of the epiphysis. It is these spaces where we aim to induce chondrogenic differentiation and thus induce endochondral ossification to grow taller.
"That is, a pressure gradient in the medullary cavity generates oscillatory fluid flow in the porous bone cortex."<-and fluid flow into the spongy bone spaces of the epiphysis.
"Modulation of the intramedullary pressure with knee loading is exerted throughout the length of the tibia and the femur."<-the epiphysis is part of the entire length thus knee loading like by LSJL alters pressure in the epiphysis.
"Proinflammatory cytokines such as IL-1β upregulate the expression and activity of MMP-1 and MMP-13. It has been shown using cultured chondrocytes that mechanical stimulation, given in a form of fluid flow shear stress, can suppress the IL-1β-induced upregulation of MMP-1 and MMP-13. In accordance with those in vitro results, joint motion in vivo is able to reduce inflammatory responses in a murine collagen-induced arthritis model. Additionally, in an antigen-induced arthritis model in rabbits, continuous passive motion suppressed transcription of IL-1β and synthesis of inflammatory mediator COX-2 and MMP-1. These mechanical signals also induced IL-10 synthesis, suggesting that moderate joint loading can generate anti-inflammatory signals."<-there are good and bad MMPs for height growth. MMP-1 and MMP-13 seem to be bad for height growth.
"When knee loading was applied to one leg, the loaded tibia and femur were reported to be longer than the non-loaded contralateral bones. In response to knee loading, the number of cells in the growth plate of the proximal tibia increased and their cellular shape was altered."<-If LSJL increases the number of cells in the growth plate by differentiation of stem cells into chondrocytes than LSJL will work in adults as well. It's possible that during knee loading only chondrocyte proliferation was increased but chondrocytes have a finite proliferative capacity and an increase in chondrocyte proliferation without increasing stem cell differentiation into chondrocytes should accelerate the the transition of proliferating chondrocytes into hypertrophic chondrocytes and not the number of cells in the growth plate.
"Homeostasis of the articular cartilage is affected through interactions with the subchondral bone underneath the cartilage. Both MMPs and ADAMTS need to be post-translationally activated, and this activation process is regulated by many factors including MMPs themselves and many proteoglycans."<-Thus loading of the articular cartilage may itself play a role in the height gain by triggering a response in the subchondral bone in response to the stimulation of the articular cartilage.
Here's another study involving Hiroki Yokota that mentions that lateral joint loading modality:
Mechanical Loading: Bone Remodeling and Cartilage Maintenance
"Because a tight coupling exists between cartilage and bone, alterations in one tissue can affect the other[So an increase in TGF-Beta expression in bone can affect cartilage growth]. Bone marrow lesions are often associated with an increased risk of developing cartilage defects, and changes in the articular cartilage integrity are linked to remodeling responses in the underlying bone. Although mechanisms regulating the maintenance of these two tissues are different, compelling evidence indicates that the signal pathways crosstalk, particularly with the Wnt pathway[loading the cartilage may also have an impact on the bone enabling the stimulation of cartilage growth within bone].."
Here's the section that mentions LSJL:
"Joint Loading applies moderate lateral loads to bone as well as joint tissues including articular cartilage and synovium. In the knee loading modality, loads are transmitted not only to the distal femur and the proximal tibia but also to the articular cartilage of the femur and tibia. [LSJL] is capable of stimulating bone remodeling throughout the lengths of the femur and the tibia, as well as suppressing the expression and activities of matrix metalloproteinases (MMPs) in the articular cartilage. A joint loading induces a periodic alteration in the pressure in the medullary cavity[it's this alteration in pressure in the epiphysis that we hope induces chondrogenic differentiation] and activates a Wnt signaling pathway in bone."
"Moderate loading of the articular cartilage generates mechanical signals that increase the synthetic activities of the chondrocyte while suppressing its catabolic actions"<-moderate loading is prochondrogenic so it'll make it easier for stem cell in the marrow to differentiate into chondrocytes. So you should definitely do some form of moderate loading in addition to LSJL.
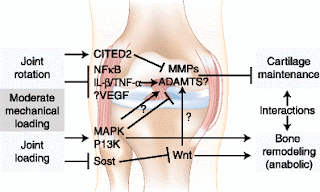
Note that joint rotation may increase VEGF which is important for the formation of cartilage canals. Joint rotation may help augment the effects of LSJL.
"Mechanical stimulation also suppresses cell death through signaling pathways including tumor necrosis factor-α (TNF-α) and Wnt"<-normal mechanical stimulation may help you grow taller through normal height development by suppressing TNF-alpha.
"CITED2 expression is increased by moderate flow shear (5 dyn/cm2), intermittent hydrostatic pressure (1–5 MPa)[thus CITED2 may be stimulated by LSJL], and joint motion. The induction of CITED2 in vivo by joint motion loading was correlated with the downregulation of MMP-1 and the maintenance of cartilage matrix integrity"
"CITED2 suppresses MMP-1 expression by preventing MMP transactivator Ets-1 from recruiting limiting amounts of co-activator p300 to the MMP-1 promoter"
"Moderate loading can block interleukin (IL)-1β–induced transcriptional activity of nuclear factor-κB (NF-κB) by interfering with multiple steps in the NF-κB signaling cascade. High-amplitude loading activates NF-κB, which regulates expression of proinflammatory cytokines and mediators such as nitric oxide synthase (NOS-2), cyclooxygenase 2 (COX-2), MMPs, TNF-α, and IL-1β. NF-κB also controls the differentiation or activity of other skeletal cell types, including osteoclasts, osteoblasts, and osteocytes"<-NF-kappaB stimulates chondrogenesis so maybe high-amplitude loading is a good thing.
"Mechanical overloading also stimulates expression of vascular endothelial growth factor (VEGF), which appears to be involved in the induction of MMP-1, −3, and −13 expressions"<-Note that LSJL does upregulate MMP-3. So it's possible that LSJL does stimulate VEGF expression.
"Quickly after osteocyte stimulation, there is an increase in intracellular Ca2+ concentrations and the release of adenosine triphosphate. These events are followed by the participation of secondary messengers prostaglandin and nitric oxide. Further downstream are elements of the Wnt pathway, which regulate bone formation and bone remodeling by promoting osteoblast proliferation and differentiation"<-Note that this release in adenosine triphosphate(ATP) can stimulate chondrocytes as well.
Wnt activation may reduce COL2A1 levels.
Here's a study not directly written by H. Yokota but was cited as being highly influential on the previous study:
Mechanical loading, cartilage degradation, and arthritis
"Moderate mechanical loading maintains the integrity of articular cartilage; however, both disuse and overuse can result in cartilage degradation. In instances of cartilage breakdown, inflammatory cytokines such as interleukin-1 beta and tumor necrosis factor-alpha stimulate the production of matrix metalloproteinases (MMPs) and aggrecanases (ADAMTSs), enzymes that can degrade components of the cartilage extracellular matrix. In order to prevent cartilage destruction, tremendous effort has been expended to design inhibitors of MMP/ADAMTS activity and/or synthesis. Accumulating evidence suggests that physiologic joint loading helps maintain cartilage integrity."
"There appears to be a critical threshold of 15–20 megapascals (MPa) for cell death and collagen damage due to a single impact load in bovine cartilage explants"<-15 MPa is incredibly high. I think we'd be lucky to get 2 MPa at most with LSJL style loading. In one study they found that 25lbs of loading increased Hydrostatic pressure by 12-14mmHg. There are 7500mmHg in one MPa. The smallest MPa listed as damaging is 5 MPa in the article.
"The range of nonphysiological load intensities in vivo should be greater than those reported in these in vitro studies."<-Due to distribution of force in a living organism thus the threshold for a damaging amount of pressure should be higher than 5MPa.
"The MMP family consists of the collagenases, (MMPs 1, 8, and 13) which degrade collagens types I, II, and III, the gelatinases (MMPs 2 and 9), which target denatured collagen, the stromelysins (MMPs 3, 7, 10, and 11), which degrade several ECM proteins and are involved in proenzyme posttranslational activation, the membrane-type MMPs (MT-MMP 1–4), and a diverse subgroup including MMPs 12, 20, and 23."<-LSJL downregulates MMP-1 which is good as you don't want degradation of type II collagen. You may want degradation of type I collagen however to make room for chondrogenesis. LSJL upregulates MMP-3 although whether this is beneficial is unknown.
"LIPUS promotes synthesis of several matrix components in chondrocytes in vitro, including type II collagen, type X collagen, and aggrecan. LIPUS also increased production of type II collagen in an experimental OA rat model and ameliorated histological cartilage damage when compared to untreated groups."<-Since LIPUS stimulates Type II Collagen and Aggrecan it too can help stimulate chondrogenesis of mesenchymal stem cells.
"Mechanical overloading stimulates expression of VEGF, which appears to be necessary for mechanically induced MMP-1, -3, and -13 expressions"<-Since LSJL upregulates MMP-3 and VEGF is necessary to the mechanically induced upregulation of MMP-3, it is logical that LSJL increases VEGF fashion possibly in a non-estrogen mediated fashion.
"Epigenetic regulation includes the activation of normally silent genes through DNA hypomethylation, which allows for an open chromatin structure, or silencing of normally expressed genes through DNA hypermethylation, which prevents access of transcription factors to their promoter."<-DNA hypomethylation could be used to activate tall genes in normal individuals? It is hard to control specific gene methylation however.
"Gene transcription can also be regulated by deacetylation through histone deacetylases (HDACs)."
"There is evidence in adult endothelial cells that shear stress regulates gene expression. Shear stress at 10 dyn/cm2/sec modifies core histones H3 and H4 in human umbilical vein endothelial cells."<-Shear stress modifies gene expression. It's not bone or cartilage cells but it shows that shear stress can modify gene acetylation. LSJL causes shear stress.
Here's a study involving Sun HB who seems to be working with Hiroki Yokota on LSJL related issues.
Mechanotransduction and cartilage integrity.
"Chondrocytes are able to sense and react to mechanically induced changes within the cartilage matrix[this is important for people who still have growth plates]. Chondrocyte mechanotransduction is initiated at the interface between the cell membrane and extracellular matrix, and the processing of these mechanical signals involves mechanoreceptors such as ion channels and integrins. Membrane stretch, a condition that chondrocytes experience during compression or during hypo-osmotic conditions that cause swelling, activates potassium channels. The function of ion channels in chondrocyte membranes is not clear, but they may be involved in chondrocyte functions such as cell proliferation and matrix secretion[so ion channels are involved in anabolic therefore height increasing functions]. Integrins are heterodimeric transmembrane receptors consisting of α and β subunits and interact with cytoskeletal proteins such as fibronectin, vitronectin, and osteopontin. Mechanical stimulation of human chondrocytes increases expression of aggrecan and decreases MMP-3{upregulated in LSJL} gene expression in a pathway involving the α5β1 integrin and IL-4 release. However, this response to mechanical stimulation is absent in chondrocytes derived from OA cartilage, suggesting abnormal chondrocyte signaling may be involved in OA disease progression[this may be one of the reasons why osteoarthritis doesn't help make you taller]"
So growth plates are capable of anabolic responses in response to mechanical load. OA however involves endochondral ossification so maybe mechanical stimulation doesn't effect chondrocytes undergoing endochondral ossification such as growth plate chondrocytes.
"One transcriptional regulator that appears to play a crucial role in cartilage homeostasis is CITED2 (CBP/p300-interacting transactivator with ED-rich tail 2). CITED2 is a transcriptional coregulator that does not bind DNA directly. It positively regulates transcription by recruiting CBP (cAMP-responsive element-binding protein) and p300 to interact with other DNA-binding transcription factors such as Lhx2, PPARα, PPARγ, Smad 2, and TFAP2. CITED2 also negatively regulates target genes by competing for CBP/p300 binding with transcription factors including Ets-1, NF-κB, HIF-1α, STAT2, and p53. Through these mechanisms, CITED2 is able to regulate many cellular processes such as embryonic development, cell proliferation, inflammation, and matrix turnover."
"With regard to cartilage integrity, CITED2 expression in chondrocytes in vitro is increased by moderate intensities of flow shear and intermittent hydrostatic pressure[LSJL induces both these things but does flow shear and hydrostatic pressure stimulate CITED2 release in stem cells?] (IHP) and in chondrocytes in vivo by joint motion. Increased CITED2 expression in vivo correlated with the maintenance of cartilage integrity and the suppression of collagenase MMP-1, suggesting the anticatabolic effects of physiologic joint loading were mediated by CITED2. CITED2 suppresses MMP-1 transcription by competing with MMP transactivator Ets-1 for binding to its coactivator p300. In addition to MMP-1, Ets-1 binds to the promoter regions of other MMPs including MMP-2, -3, -8, -9, and -13. Therefore, it is likely CITED2 may regulate additional MMPs through a similar manner.
Upstream of CITED2, moderate IHP loading phosphorylated p38δ, which was required for the transactivation of CITED2[stem cells have p38 meaning that hydrostatic pressure likely can stimulate CITED2 activation in stem cells too]. p38 belongs to the MAP kinase family, which is activated in response to mechanical stresses. While moderate loading activated p38δ and CITED2, high levels of IHP phorphorylated p38α and MMP-1, but not CITED2. This may explain why CITED2 is specifically activated by moderate loading and also suggests that p38α is involved in the upregulation of MMP-1[MMP-1 is catabolic however very high levels of hydrostatic pressure has been shown to be anabolic in inducing chondrocyte differentiation]. Different members of the p38 family may act as a “mechanosensitive switch” in chondrocytes, which act to upregulate or downregulate MMP expression based on the mechanical loading regimes. The evidence that CITED2 is inducible by IL-4 and may interact with components of NF-κB, suggests a potential role of CITED2 as a central mediator in these mechanotransduction pathways involved in maintaining cartilage integrity"
So CITED2 competes with MMP-1 a catabolic protein. So moderate levels of hydrostatic pressure may be better with existing growth plates to activate CITED2 and not MMP-1 but high levels of hydrostatic pressure may be better to induce new chondrocyte differentiation where growth plates are absent.
Here's a study that shows that CITED2 may be inhibitory towards new cartilage and bone growth:
Identification of CITED2 as a negative regulator of fracture healing.
"The transcription regulator CITED2 (CBP/p300-Interacting-Transactivator-with-ED-rich-tail-2) is known to suppress genes mediating angiogenesis and extracellular matrix (ECM) remodeling. We tested the hypothesis that CITED2 functions in bone fracture healing by suppressing the expression of genes critical to ECM remodeling, angiogenesis and osteogenesis, importantly the matrix metalloproteinases (MMPs). Three hours following mandibular osteotomy[removal of bone] or sham surgery of adult rats, osteotomy fronts were harvested and the expression of CITED2 and genes associated with fracture healing was ascertained by quantitative PCR. In parallel, gain-of-function studies examined the effect of overexpressing CITED2 on the expression and activity of several MMPs. In the fractured mandible, CITED2 expression was inversely related to the expression of MMP-2, -3, -9, -13, VEGF, HIF-1alpha, M-CSF, RANK-L, and OPG[Hypoxia inducible Factor-1 and CSF are highly anabolic factors for endochondral ossification]. Consistent with this, the over-expression of CITED2 in osteoblasts inhibited the expression and activity of MMP-2, -3, -9, and -13."
Since fracture healing often involves endochondral ossification, perhaps CITED2 inhibits new endochondral ossification from occurring. However, fracture healing usually requires catabolic activity to remodel bone. New height growth requires catabolic activity to make way for the new growth plates. However, CITED2 may also be important to discourage catabolism of chondrogenic components formed in the bone.
"Early fracture healing is characterized by the initial formation of cartilage tissue in the callus, which is then resorbed by MMPs to allow for vascular invasion with the eventual replacement of cartilage with osseous tissue[so perhaps allowing for CITED2 may allow grow plates to stay active longer]. MMPs 9 and 13 are critical to normal skeletal development; most notably, MMP-9 deficiency delays fracture healing with poor cartilage resorption and impaired capillary and chondroclast invasion. MMP-2, in contrast, participates in cartilage degradation, while MMP-3 activates pro-MMP-9 during wound repair"
So inhibiting CITED2 may aid in the formation of new growth plates but CITED2 may help preserve existing growth plates.









