inversions
purpose and passion
- Joined
- Mar 23, 2025
- Posts
- 11,347
- Reputation
- 25,123
bro the questions are literally piss easyI'm OCR-A
Tbh it's not that hard, the savemyexams Q's were easy
View attachment 4363302
But the end of chapter Q's in the book are inpossible
View attachment 4363309
Obviously not impossible but confusing and harder to answer than the sme ones


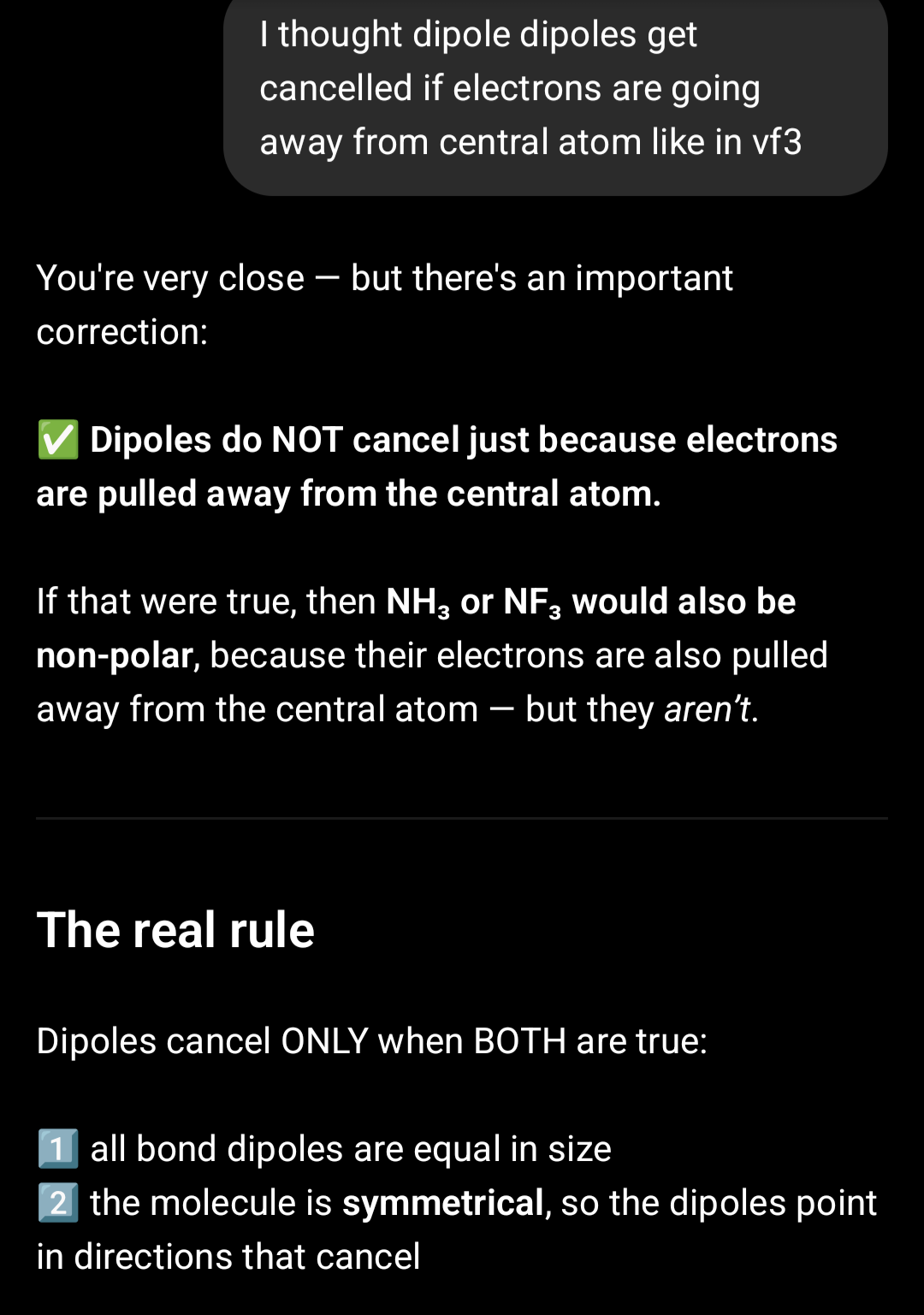
 non polar obviously because fluorine is more electronegative but why does it contain polar bonds
non polar obviously because fluorine is more electronegative but why does it contain polar bonds