D
Deleted member 230154
Iron
- Joined
- Sep 17, 2025
- Posts
- 44
- Reputation
- 36
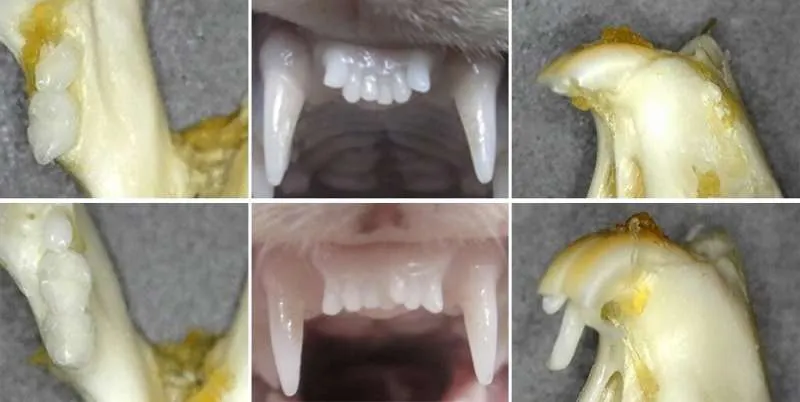
Also keep in mind they pulled the tooth of a mice and a ferret in which both grew a brand new tooth after the previous one was extracted.
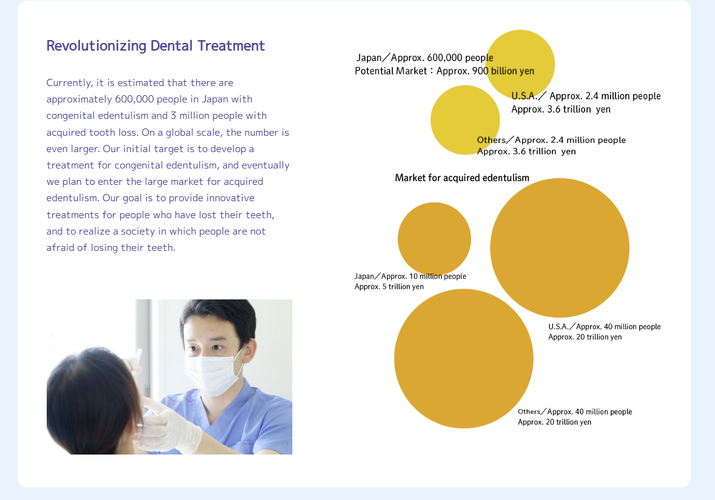
Toregem BioPharma's investigational drug TRG035, an anti-USAG-1 antibody, is currently in Phase I clinical trials in Japan, primarily focused on treating severe congenital hypodontia, a rare condition where six or more permanent teeth are missing from birth. While the initial trials target congenital cases, the company's long-term vision includes expanding research into acquired tooth loss caused by trauma, aging, or periodontal disease. The development of TRG035 is supported by Japan’s Agency for Medical Research and Development (AMED) and has received orphan drug designation from Japan’s Ministry of Health, Labor
 and Welfare, which provides incentives to accelerate development. The drug works by neutralizing the USAG-1 protein, which inhibits natural tooth development, thereby reactivating dormant tooth buds through the BMP and Wnt signaling pathways. Although the current trials are focused on congenital cases, Toregem BioPharma aims to explore its potential for acquired tooth loss in future phases, with the goal of offering regenerative solutions for adults as well. The company plans to collaborate with medical institutions and universities worldwide to advance this research. If successful, TRG035 could become the first biological therapy capable of regenerating teeth in humans, potentially redefining dental care globally. The projected timeline for general availability is around 2030, pending successful completion of ongoing and future clinical trials.
and Welfare, which provides incentives to accelerate development. The drug works by neutralizing the USAG-1 protein, which inhibits natural tooth development, thereby reactivating dormant tooth buds through the BMP and Wnt signaling pathways. Although the current trials are focused on congenital cases, Toregem BioPharma aims to explore its potential for acquired tooth loss in future phases, with the goal of offering regenerative solutions for adults as well. The company plans to collaborate with medical institutions and universities worldwide to advance this research. If successful, TRG035 could become the first biological therapy capable of regenerating teeth in humans, potentially redefining dental care globally. The projected timeline for general availability is around 2030, pending successful completion of ongoing and future clinical trials.
Toregem BioPharma's investigational drug TRG035, an anti-USAG-1 antibody, is currently in Phase I clinical trials in Japan, primarily focused on treating severe congenital hypodontia, a rare condition where six or more permanent teeth are missing from birth. While the initial trials target congenital cases, the company's long-term vision includes expanding research into acquired tooth loss caused by trauma, aging, or periodontal disease. The development of TRG035 is supported by Japan’s Agency for Medical Research and Development (AMED) and has received orphan drug designation from Japan’s Ministry of Health, Labor