7evenvox22
.
- Joined
- May 29, 2025
- Posts
- 599
- Reputation
- 1,495
Thread Song:
Introduction:
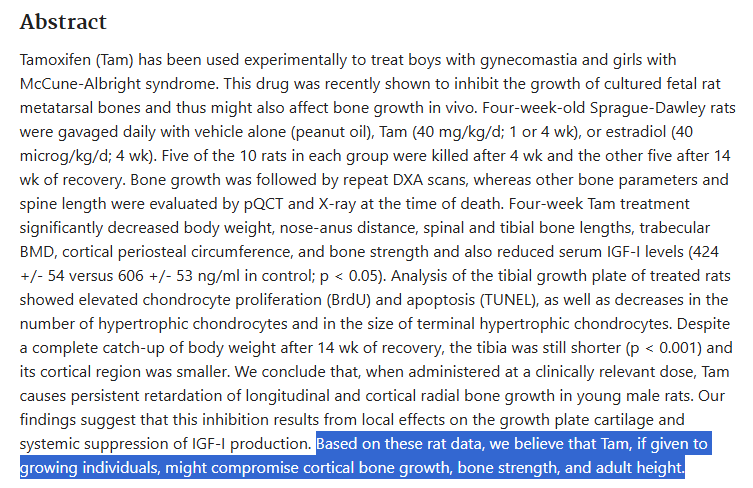
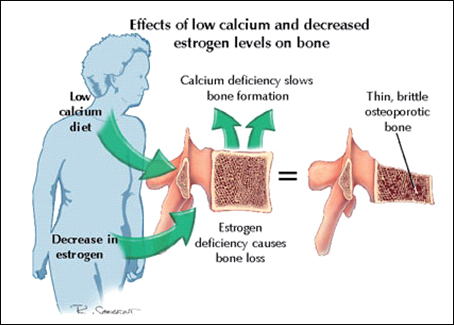
Estrogen plays a paradoxical role in development. It’s one of the most important hormones for proper skeletal, neurological, and metabolic maturation, yet it’s also the single biggest factor responsible for growth plate closure. Globally suppressing estrogen through aromatase inhibitors can indeed prolong growth, but it comes at the cost of decreased bone quality, impaired brain development, and systemic dysfunction.
So, the real question is:
Can you selectively inhibit estrogen locally in the growth plates without touching it elsewhere?

The Case of Tamoxifen (Tamex):
Tamoxifen has long been studied as a selective estrogen receptor modulator (SERM), a compound that can act as an antagonist in some tissues and an agonist in others. However, its effects on bone and the growth plate are far more nuanced than people realize.
In the growth plate, Tamoxifen exhibits antagonistic properties, meaning it inhibits estrogen-mediated closure. Yet, paradoxically, it preserves or even promotes bone health in other tissues. This makes it a theoretically perfect candidate for localized modulation, at least on paper.
The issue?
Tamoxifen also lowers IGF-1 levels significantly and exhibits ambiguous estrogenic activity in other tissues, including the liver and brain. This systemic interference makes it suboptimal for precise growth modulation.

The Real Key: Tamoxifen’s Metabolites
Most people overlook the fact that Tamoxifen itself isn’t the true active compound responsible for these effects. Its metabolites are.
Among them, Endoxifen stands out. It binds to the estrogen receptor with over 100x the affinity of Tamoxifen itself, and its activity profile is much more selective. Another metabolite, Norendoxifen, has aromatase-inhibiting properties, but it’s Endoxifen that holds the most promise for targeted growth plate modulation.
ERα Modulation: The Core Mechanism
The estrogen receptor alpha (ERα) has two major activation domains:
- AF-1: Promotes bone formation and maintains bone health.
- AF-2: Drives growth plate maturation and closure.
For controlled linear growth, the goal is simple, inhibit AF-2 while keeping AF-1 active.
Endoxifen accomplishes exactly that. It selectively antagonizes AF-2 while leaving AF-1 intact. Even more impressively, it promotes ERα degradation and blocks non-genomic signaling cascades like PKC/SET, which further drive hypertrophy and closure.
This dual action means you’re effectively shutting down the molecular switch that closes the plate, while preserving the receptor functions responsible for bone quality and skeletal strength.

Why Endoxifen > Tamoxifen
- Higher ER Affinity: 100x stronger binding than Tamoxifen.
- Selective AF2 Antagonism: Inhibits closure while maintaining AF1-mediated bone integrity.
- Minimal IGF-1 Suppression: Much weaker hepatic ER signaling.
- Limited Central Activity: Reduced impact on neuroendocrine feedback.
Together, these make Endoxifen far more promising for local modulation of growth plates without the widespread drawbacks of full systemic estrogen blockade.

Metabolic Considerations: CYP26D & Enzymatic Variability
The enzyme CYP26D is primarily responsible for Endoxifen metabolism. Anything that inhibits this enzyme will drastically alter the ratio of Tamoxifen metabolites, which can completely shift how the drug behaves in the body.
This is critical because several commonly used compounds can inhibit CYP26D, unintentionally changing Endoxifen’s balance and potency. Furthermore, species differences (rat vs human) explain why preclinical results often conflict with human data. These enzymes function differently across species, leading to vastly different outcomes in growth plate modulation.
Summary:
- Estrogen drives growth plate closure primarily via ERα AF2 activation.
- Tamoxifen antagonizes this pathway, but its systemic ambiguity limits usefulness.
- Endoxifen, its dominant metabolite, selectively blocks AF2 while leaving AF1 active, maintaining bone integrity while delaying closure.
- CYP26D determines metabolite balance inhibition alters effects.
- Localized estrogen modulation is theoretically possible, and Endoxifen appears to be one of the most promising routes toward achieving it.
If you enjoyed this thread, bookmark it. I’ll be releasing a follow-up soon, diving deeper into how to exploit tissue-selective receptor modulators to fine-tune estrogenic signaling for skeletal development.

I apologize for my horrendous formatting . I seriously need someone to help me with it for my future threads.
. I seriously need someone to help me with it for my future threads.
Introduction:
Estrogen plays a paradoxical role in development. It’s one of the most important hormones for proper skeletal, neurological, and metabolic maturation, yet it’s also the single biggest factor responsible for growth plate closure. Globally suppressing estrogen through aromatase inhibitors can indeed prolong growth, but it comes at the cost of decreased bone quality, impaired brain development, and systemic dysfunction.

So, the real question is:
Can you selectively inhibit estrogen locally in the growth plates without touching it elsewhere?

Surprisingly, yes, there are multiple ways to modulate estrogenic activity at the tissue level without globally crashing E2.
The Case of Tamoxifen (Tamex):
Tamoxifen has long been studied as a selective estrogen receptor modulator (SERM), a compound that can act as an antagonist in some tissues and an agonist in others. However, its effects on bone and the growth plate are far more nuanced than people realize.
In the growth plate, Tamoxifen exhibits antagonistic properties, meaning it inhibits estrogen-mediated closure. Yet, paradoxically, it preserves or even promotes bone health in other tissues. This makes it a theoretically perfect candidate for localized modulation, at least on paper.

The issue?
Tamoxifen also lowers IGF-1 levels significantly and exhibits ambiguous estrogenic activity in other tissues, including the liver and brain. This systemic interference makes it suboptimal for precise growth modulation.

The Real Key: Tamoxifen’s Metabolites
Most people overlook the fact that Tamoxifen itself isn’t the true active compound responsible for these effects. Its metabolites are.
Among them, Endoxifen stands out. It binds to the estrogen receptor with over 100x the affinity of Tamoxifen itself, and its activity profile is much more selective. Another metabolite, Norendoxifen, has aromatase-inhibiting properties, but it’s Endoxifen that holds the most promise for targeted growth plate modulation.

ERα Modulation: The Core Mechanism
The estrogen receptor alpha (ERα) has two major activation domains:
- AF-1: Promotes bone formation and maintains bone health.
- AF-2: Drives growth plate maturation and closure.
For controlled linear growth, the goal is simple, inhibit AF-2 while keeping AF-1 active.
Endoxifen accomplishes exactly that. It selectively antagonizes AF-2 while leaving AF-1 intact. Even more impressively, it promotes ERα degradation and blocks non-genomic signaling cascades like PKC/SET, which further drive hypertrophy and closure.
This dual action means you’re effectively shutting down the molecular switch that closes the plate, while preserving the receptor functions responsible for bone quality and skeletal strength.

Why Endoxifen > Tamoxifen
- Higher ER Affinity: 100x stronger binding than Tamoxifen.
- Selective AF2 Antagonism: Inhibits closure while maintaining AF1-mediated bone integrity.
- Minimal IGF-1 Suppression: Much weaker hepatic ER signaling.
- Limited Central Activity: Reduced impact on neuroendocrine feedback.
Together, these make Endoxifen far more promising for local modulation of growth plates without the widespread drawbacks of full systemic estrogen blockade.

Metabolic Considerations: CYP26D & Enzymatic Variability
The enzyme CYP26D is primarily responsible for Endoxifen metabolism. Anything that inhibits this enzyme will drastically alter the ratio of Tamoxifen metabolites, which can completely shift how the drug behaves in the body.
This is critical because several commonly used compounds can inhibit CYP26D, unintentionally changing Endoxifen’s balance and potency. Furthermore, species differences (rat vs human) explain why preclinical results often conflict with human data. These enzymes function differently across species, leading to vastly different outcomes in growth plate modulation.
Summary:
- Estrogen drives growth plate closure primarily via ERα AF2 activation.
- Tamoxifen antagonizes this pathway, but its systemic ambiguity limits usefulness.
- Endoxifen, its dominant metabolite, selectively blocks AF2 while leaving AF1 active, maintaining bone integrity while delaying closure.
- CYP26D determines metabolite balance inhibition alters effects.
- Localized estrogen modulation is theoretically possible, and Endoxifen appears to be one of the most promising routes toward achieving it.
If you enjoyed this thread, bookmark it. I’ll be releasing a follow-up soon, diving deeper into how to exploit tissue-selective receptor modulators to fine-tune estrogenic signaling for skeletal development.

I apologize for my horrendous formatting
 . I seriously need someone to help me with it for my future threads.
. I seriously need someone to help me with it for my future threads.