What We know
Bone density increase through remodeling managed by osteocytes who inhibit sclerostin thus lifting the suppression on the Wnt/β-catenin pathway. This activation prompts osteoblast differentiation through RUNX2 and localized IGF-1 resulting in enhanced osteoid production and its mineralization into hydroxyapatite. Simultaneously a lowered RANKL/OPG ratio diminishes osteoclastic resorption. The end result is a more compact and robust bone that responds to mechanical hormonal and metabolic cues.Trenbolone increases bone mass by binding to androgen receptors on osteoblasts, directly stimulating their proliferation and RUNX2 transcription. It elevates local IGF-1 sensitivity here, enhancing osteoblast differentiation and osteoid mineralization. it also reduces osteoclastic activity via a lowered RANKL/OPG ratio.
Its also the case for every androgen, but since Trenbolone has a binding affinity 3x greater than Testosterone on the androgen receptor, it can be microdosed effectivly
STUDIES
In this study orchiectomized (castrated) rats were either given 7mg of testosterone enanhate or 1mg of Trenbolone Enanhate per week for 5 weeks intramuscularly.They showed reduced loss or even improvement of trabecular bone mineral density compared to control groups.
in this other study obese and castrated rats received 2.0/mg/kg/day of testosterone (HF/HS + ORX +TEST, n=10) or 2.0/mg/kg/day trenbolone (HF/HS+ORX+TREN, n=10) oral or subcutaneous chronic dosing
Both testosterone and trenbolone treatment effectively restored the adverse changes in femoral bone mineral density and structure caused by obesity and hypogonadism
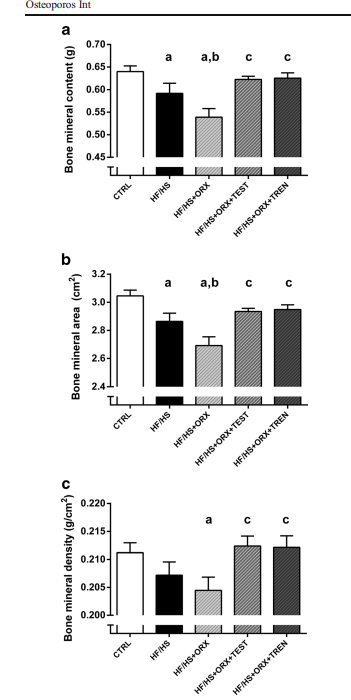
However, when applying standard body-surface-area (BSA) allometric scaling, the rodent dose translates to approximately 24 mg per kilogram per day in humans — a massive exposure for an adult male. But the previous study demonstrated that even a minimal dose of 1 mg per week was sufficient to attenuate trabecular BMD loss.
LIMITS
While rodent data clearly demonstrate that trenbolone and testosterone can prevent trabecular bone loss through AR-mediated stimulation of RUNX2 Wnt/β-catenin activation and suppression of RANKL-driven resorption the translational relevance to humans remains limited. Human bones particularly cortical and craniofacial structures exhibits much slower remodeling dynamics. Furthermore rodent dosing regimens whether 1 mg/week injections or 2 mg/kg/day continuous delivery cannot be linearly extrapolated to human physiology due to species differences in pharmacokinetics surface-area scaling and AR occupancy thresholds. If we are Based on the rats studies, the minimal effective dose of trenbolone preventing trabecular BMD loss was 1 mg/week in ORX rats. Using standard body surface area allometric scaling this translates to approximately 0.65 mg/kg/week in humans. For a 75 kg adult male, this corresponds to a theoretical weekly dose of about 50 mg IM. However, the exposure in these studies was limited to just a few weeks, so the long-term impact on bone mass remains unknown. It is plausible that a longer duration of androgenic stimulation could yield even greater increases in overall BMD

