ihatemySOST
Bronze
- Joined
- Sep 5, 2025
- Posts
- 479
- Reputation
- 770
This thread contains misinformation.
Introduction:
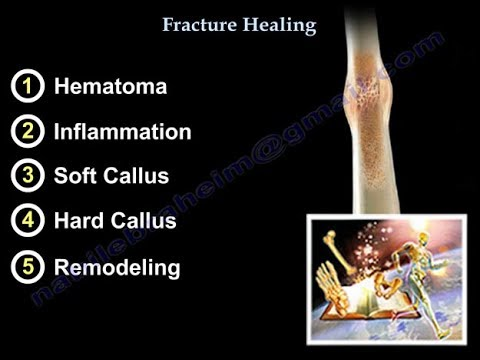
Bone smashing is one of the most popular methods on this forum and in fact not just on this forum but in the looksmaxing community in general.The idea behind it is that hitting the bone will create mechanical stress on it which will lead to an increase in its size and people usually refer to Wolff’s law to prove this point.But this is completely illogical.First Wolff never mentioned that bone size increases due to mechanical stress all he mentioned was bone density.Second Wolff stated that physiological mechanical stress resulting from daily activities is what causes bone adaptation not random hits on the bone [1].So it’s clear that interpreting the effectiveness of bone smashing based on a loosely defined law created by a German surgeon more than 120 years ago and misinterpreting it is not proof at all of the effectiveness of hitting your facial bones to increase their size.Does that mean bone smashing is ineffective?Well not exactly.Bone smashing might actually be the only scientifically proven method to increase bone size but certainly not based on a random law written by a German scientist and misinterpreted by kids.There’s something much deeper than that and it’s called “subperiosteal hematoma ossification.”You may have already heard this term before from my previous post but to be honest the explanation itself was one thing while the organization of information and the way it was presented were somewhat off even if the idea I wanted to convey was somewhat clear
Note before starting the explanation everything I say is 100% based on science all sources have already been included by the time you’re reading this post)Okay what is a subperiosteal hematoma?Simply it’s a phenomenon that occurs due to blood leaking between the periosteum and the bone layer because of a rupture in the blood vessels which leads to the periosteum being lifted off the bone forming a shape that resembles a “mountain plateau.”There are several causes that can lead to the formation of a subperiosteal hematoma but the most common and scientifically agreed upon cause is direct hits or trauma to the bone.Why?Because it leads to the rupture of blood vessels then the leakage of blood between the periosteum and the bone which results in the lifting of the periosteum layer off the bone (See figure 1 to understand)
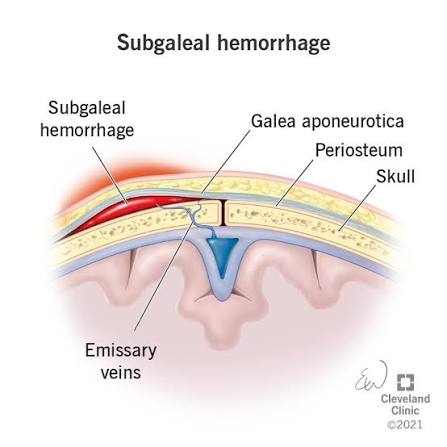
(Figure 1 you can see a subperiosteal hematoma and a partial elevation or separation of the periosteum from the bone)
Okay I think the concept of subperiosteal hematoma is already clear but what is its ossification?How does it ossify?And what is the scientific explanation for that?Well there are four hypotheses that explain it logically and I will explain them (of course everything is scientifically based) then I will show documented evidence of massive bone hypertrophy that occurs due to the ossification of a subperiosteal hematoma
The first theory the inflammatory environment (the most important factor):When a subperiosteal hematoma occurs as a result of an injury or a direct blow to the bone the blood vessels connecting the periosteum and the bone rupture leading to blood accumulation and the spread of red blood cells.After some time these cells coagulate and cause the release of a group of inflammatory factors from the blood components such as platelets macrophages and other immune cells.These factors include cytokines such as IL-1β IL-6 TNF-α as well as chemokines like MCP-1 and growth factors such as TGF-β VEGF BMPs and cox2.These factors create a favorable environment for stimulating bone formation through several biological mechanisms.First the inflammatory cytokines help attract mesenchymal stem cells (MSCs) from the bone marrow and the inner layer of the periosteum which is very rich in MSCs to the injury site.These stem cells migrate toward the affected area due to chemokine signals and are activated by cytokines and inflammatory mediators like IL-6 and TGF-β.Upon arrival the stem cells begin differentiating into osteoblasts thanks to stimulation by growth factors such as BMPs and TGF-β leading to the secretion of the initial bone matrix composed of collagen and calcium salts.At the cellular level these factors activate signaling pathways such as SMAD MAPK and JAK/STAT.The importance of inflammatory factors can be easily proven by the fact that when inflammatory factors are removed there is a severe delay in bone healing
The second theory change in electrical potential:When a subperiosteal hematoma occurs due to an injury or a fracture blood accumulates between the periosteum and the bone leading to mechanical pressure and electrical changes that affect bone cells and the healing process.If anyone here knows what microneedling for the skin is you probably know that the main reason for its effectiveness is not what people usually think but actually because of changes in electrical potential which makes the body believe that the skin has been torn or injured when in reality nothing happened except a change in electrical potential so the body releases growth factors to heal it.The same concept likely applies to bone.Bone has piezoelectric properties meaning it generates electrical signals when subjected to stress or strain.When blood accumulates under the periosteum it changes the mechanical stress pattern on the bone generating a local electrical potential in the affected area.This electrical potential acts as a signal that stimulates osteocytes and mesenchymal stem cells (MSCs) in the area.The stem cells respond to these electrical signals by migrating to the injury site via integrin receptors on their surface and differentiating into osteoblasts.Once these cells reach the site they begin secreting bone matrix made of collagen and calcium salts contributing to the formation of new bone.Furthermore the change in electrical stress activates signaling pathways such as the calcium pathway and MAPK leading to the opening of calcium channels in bone cells.Simply put this theory is based on the idea that the change in electrical potential tricks your body into believing that a fracture has occurred in the bone when in reality nothing has happened to it
The third theory severe oxygen deprivation:When the bone is exposed to a direct impact the blood vessels rupture leading to blood clotting which creates a highly hypoxic environment.You might think this is a bad thing but in reality it’s the opposite.Recent research shows that hypoxia plays a crucial and essential role in bone formation.The rupture of blood vessels cuts off the oxygen supply to the bone which acts as a trigger for the body to increase the expression of a gene called HIF-1α (Hypoxia Inducible Factor-1 alpha).This protein or gene is very important for osteogenic differentiation through multiple pathways.First by increasing the expression of VEGF second by enhancing the activity of the Wnt/β-catenin signaling pathway and also by upregulating BMPs.Moreover it inhibits the activity of Twist2 which leads to an increase in the gene expression of Runx2 and Osterix.Simply the hypoxic environment is ideal for the differentiation of stem cells attracted from the inner layer of the periosteum by inflammatory factors as it drives them to differentiate into osteogenic cells and form a mature and complete bone matrix at the injury site (the site of the hematoma)
The fourth theory tensile force caused by the hematoma:This hypothesis may be the most logical one since many scientists have stated that it is the most reasonable explanation for the ossification of a subperiosteal hematoma.This theory suggests that the hematoma lifts the periosteum away from the bone layer which activates the MSCs located in the inner layer of the periosteum (the cambium layer) through their mechanoreceptors such as primary cilia integrins and purinergic receptors.Researchers supported this hypothesis based on an in vitro study where stem cells extracted from the inner layer of the periosteum were subjected to tensile force (similar to what cells might experience during a hematoma) and this indeed led to an increased gene expression of Runx2 Osterix and BMP which are crucial genes and proteins responsible for driving MSCs to differentiate into osteogenic cells
In summary, hitting the bone causes the blood vessels to rupture which creates an inflammatory environment, hypoxia, changes in electrical potential, and tensile stress on stem cells, leading to their transformation into a new bone layer (often this transformation occurs directly without a cartilage template since the periosteum itself is not damaged).When repeated hits are applied continuously over months or years, a condition called chronic peripheral periostitis occurs, resulting in the formation of a prominent bony mass at the site of injury.
(Note, as I mentioned earlier everything is scientifically based, and for the scientific sources they are [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23])
Okay, is there clinical evidence proving that a subperiosteal hematoma can ossify and become bone?The answer is yes, in fact there are hundreds or even thousands of studies, evidence, and case reports supporting this.I will now present some of them and show evidence proving that the mechanism I mentioned applies to any bone in the body, and I will definitely provide supporting proof for that.
Many of you may already know about bareback rodeo, a sport that requires the rider to have their forearm directly strike the hip bone. Here is a literal excerpt from the study describing what happens: “On observing a contestant who is riding, the riding arm is noted to contact the anterior iliac crest and the heavily resined chaps (Fig. 3). The event requires strength, timing, balance, and courage. Ideally, the forearm should be tucked against the pelvis and chaps. Failure to maintain this position results in a hammering action of the forearm against the pelvis and chaps.“You don’t have to be particularly smart to understand this literally; the scientists stated that the hypertrophy that occurs is the result of direct impact or a “hammering action.”If you’re wondering about the extent of the bone growth, to be frank there was massive hypertrophy, although it varied among participants (I will explain the reasons for this variation).In one sample, the diameter of the ulna increased by 92% and the bone’s cross-sectional area by 262%, which are extremely large biological increases, especially considering participation in this sport is prohibited before age 18.Some might argue that this hypertrophy is due to muscle tension rather than direct impact on the bone, but this is incorrect for several reasons.First, many sports apply intense torsion forces to bones but do not cause even a 10% increase as observed in rodeo riders.Second, the hypertrophy occurs exclusively at the area where the ulna contacts the hip.Third, the hypertrophy was observed only in riders who did not use a saddle; the saddle acts as a barrier preventing friction and impact with the hip bone, and its absence allows direct friction and impact.Fourth, the variation in hypertrophy among participants is likely because some wore protective gear to prevent friction while others did not (see figure 2 to view the bone hypertrophy) [25].
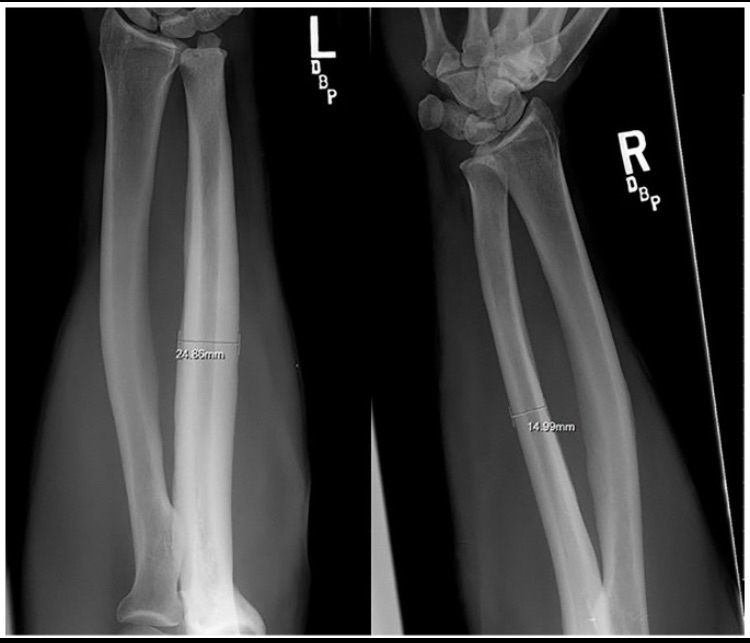
(Figure 2, an image showing bone hypertrophy, please note that the increase in diameter here was approximately 62%, but the largest increase reached 92%)
In another study with the same objective as the first but older, we can say that they found exactly the same results massive bone hypertrophy occurred in bareback rodeo riders. Fortunately, this time the supervising researcher fully agrees with my scientific explanation for this hypertrophy. I will quote directly from him: “The hypertrophy is considered by the author to be a chronic circumferential periostitis secondary to trauma.“It seems the author completely agrees with me, explaining the hypertrophy as resulting from direct trauma to the bone (the trauma from the ulna hitting the pelvis), which leads to chronic peripheral periostitis and subsequently bone hypertrophy.This study strongly supports my theory, because how else would you explain massive bone growth resulting from periosteal inflammation (as we mentioned earlier, through hematoma formation, red blood cell coagulation, and release of inflammatory factors)? Continuing in this state eventually leads to enormous bone hypertrophy.An important point is that a rodeo round actually lasts only 8 seconds, which debunks the argument that you need to spend hours daily to do bone smashing. If that were true, how do you explain the bone hypertrophy in rodeo riders who only do very limited rounds per day?Not to mention that the bone isn’t in constant impact throughout the round, probably only 2–3 seconds in total (see figure 3 to view the hypertrophy) [26]
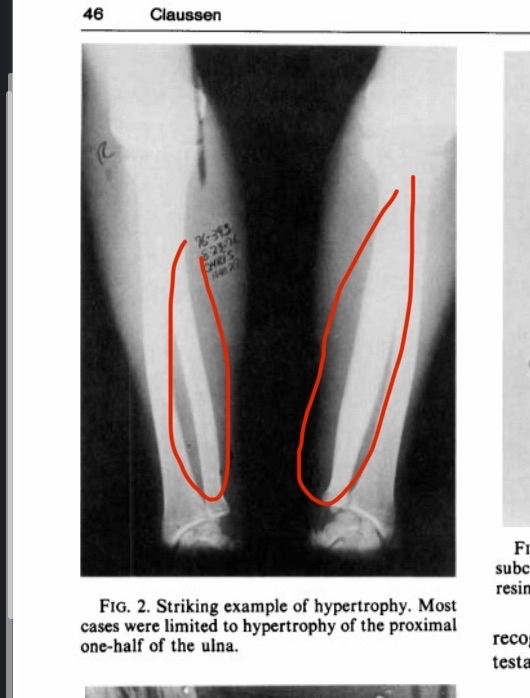
(Figure 3, bone hypertrophy resulting from practicing rodeo)
The evidence supporting my theory is not limited to these studies. In fact, there is much more. For example, a 15-year-old soccer player experienced a direct injury to his knee. What happened next? A subperiosteal hematoma formed, followed by bone ossification and the appearance of a clear bony mass on X-ray [27]. There are many similar case reports. For instance, in a lacrosse player, a sport that involves direct contact with opponents, the athlete reported pain in an area left unprotected. That area continued to experience direct hits, bleeding, and eventually bone hypertrophy. Scientists described this exactly as it happened, and indeed this was confirmed on X-ray [28].
In another case, a person fell on their ankle and was injured. After a while, a subperiosteal hematoma formed, which then ossified to become bone (we have already explained how a hematoma ossifies). I am not making this up; this is literally what the study and researchers reported [29]. In another case, a soccer defender’s humerus continued to experience direct impact during defense, leading to a subperiosteal hematoma, then ossification, and finally transformation into bone. The scientists reported that this hypertrophy was mainly due to repeated microtrauma to the bone [30].
In another individual case, a woman hit her finger on a cabinet, leading to a hematoma, which then ossified into new bone [31]. In another case, a Muay Thai boxer experienced massive bone hypertrophy clearly visible on X-ray, which scientists attributed to repeated microtrauma [32]. In another individual case, a child fell on their pelvis while skiing, and radiographs showed very dense bone hypertrophy at the injury site due to a subperiosteal hematoma (see figure 4 to view the hypertrophy) [33].
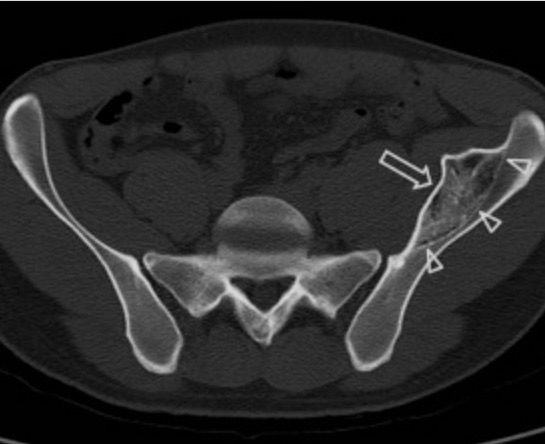
(Figure 4, an image showing a dense bony mass resulting from the periosteum being lifted off the bone layer and ossification of the hematoma)
So, it seems I have already presented many studies and case reports documenting the phenomenon I discussed, which is blood vessel rupture, followed by subperiosteal hematoma formation, and then ossification. I have also conclusively shown that this mechanism applies to all bones in the body, such as the hip, femur, tibia, hand, and fingers. Please note that there are hundreds, if not thousands, of case reports documenting the same process repeatedly. But what about the skull bones? Are there any cases documenting subperiosteal hematoma formation and subsequent ossification? The answer is yes. In fact, case reports usually show severe bone hypertrophy at the injury site, possibly because the periosteum’s attachment to the skull is looser compared to other bones, making subperiosteal hematoma formation easier.
For example, in a case of a 35-year-old woman who experienced a severe blow to her frontal bone, what happened? As expected, a subperiosteal hematoma formed, then ossified into a large bony mass. Interestingly, the growth continued even after several years. The exact scientific explanation for the continued bone growth after years from the injury is unclear, but it may be an exaggerated protective response by the body (see figure 5) [34].
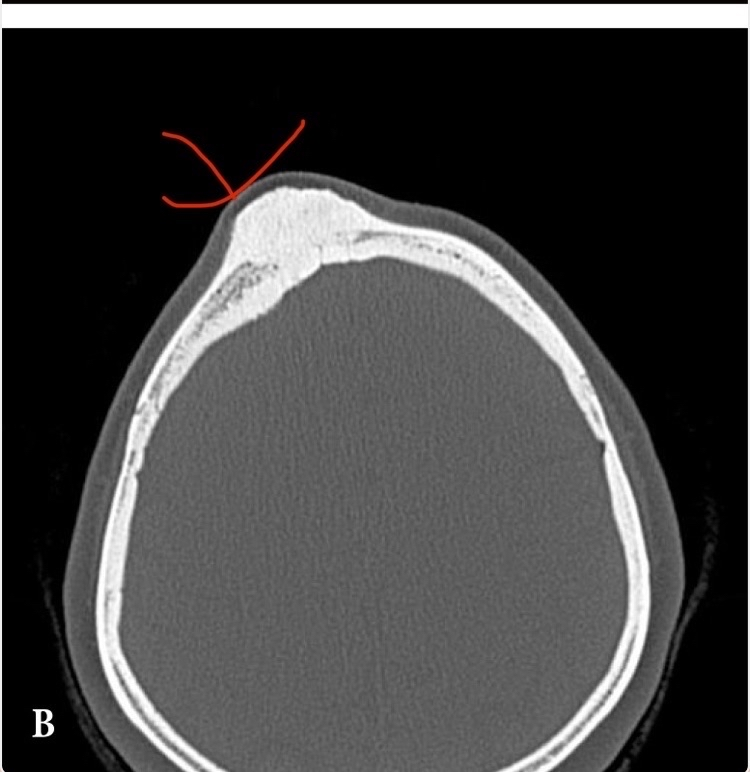
(Figure 5: a prominent bony mass at the injury site formed years after ossification of the subperiosteal hematoma)
In another case, a young child experienced a direct blow to the orbital bone while watching a match. After some time, the hematoma ossified, forming a very large bony mass at the injury site after 11 months. Scientists reported that the hypertrophy was due to the release of inflammatory factors and the tensile force applied to the periosteum [35]. In another case, a child sustained an injury to the side of the supraorbital area, and similarly, bone hypertrophy was observed at the injury site [36]. Not only that, in another case, a person received a blow to the lower jaw while playing soccer, and after some time, bone formation appeared at the injury site (see figure 6) [37].
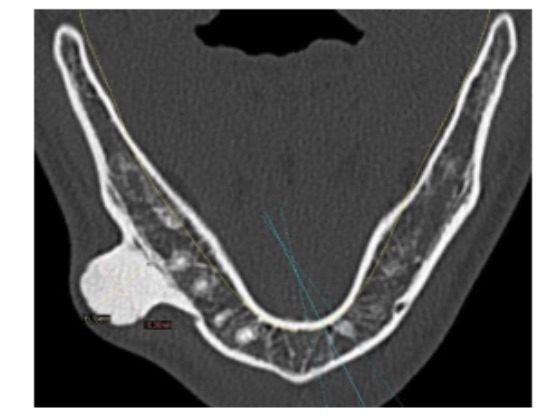
(Figure 6: a prominent bony mass in the lower jaw resulting from a direct injury to the mandible during a soccer match)
Discussion:
It seems that, without a doubt, the theory is correct. This has been documented in all cases, and most scientists usually explain the result by two factors: first, the release of inflammatory factors due to the coagulation of red blood cells; second, the tensile force applied by the hematoma on the MSCs in the periosteum. There are hundreds, if not thousands, of pieces of evidence documenting the same phenomenon.
It is also important to mention something crucial: the younger the person, the faster the bone growth. Why? This is the same reason why fractures heal much faster in children than in adults, due to the higher activity of stem cells in the inner layer of the periosteum. This means that anyone still in puberty has a golden opportunity to exploit a subperiosteal hematoma to transform it into bone.
Okay, let’s be realistic. Will the bone growth be aesthetic? The answer depends on the person. Bone growth won’t happen magically or randomly; it will simply occur where the hematoma forms, or where the blood vessels rupture. So why does bone formation often appear random on X-rays? The answer is simple: the hematoma occurred randomly because the impact was random.
Remember, the body doesn’t build bone to make you more aesthetic. It simply builds bone for protection. So regardless of whether the bone grows aesthetically or not, you are in control. You determine when and where the blood vessels rupture. I can easily prove that the hematoma can be aesthetic. When people here perform bone smashing, they often report that the hematoma that forms is aesthetic.
Logically, the blood will ossify only at the injury site. Nothing magical grows randomly unless the hematoma itself is random. I can also prove this by looking at rodeo riders, where bone hypertrophy is natural and not random. Why? Simply because the bone grows only at the spot receiving the impact. If growth were truly random, why do we observe that bone growth in rodeo riders is natural and even aesthetic? Anyone who thinks bone growth cannot be aesthetic must answer all the questions I just posed.
Okay, this theory refutes any argument against bone smashing. For example, it refutes the claim that you must be young for it to work, which is not true. For instance, stem cells in the periosteum of rabbits are very active at all life stages and have the ability to form bone at any age [38]. But what about humans? The answer is yes. If it weren’t, how would you explain bone healing after fractures in elderly people? The only explanation is that periosteal stem cells remain active and are reactivated during fracture, just as shown in the rabbit study. Yes, of course, the younger you are, the more active they are there is no doubt about that.
This theory also refutes the idea that direct bone impact won’t stimulate bone growth, and that you need physiological force rather than direct strikes to the bone. The theory is based on the fact that blood vessels must be ruptured for blood to escape, and direct impact on the bone is very effective at this. It also refutes the notion that bone increases in size from mechanical tension alone, because there is plenty of evidence showing that hematoma ossification has a high potential for ossification (the younger you are, the higher the chance). In reality, mechanical tension on a specific bone may primarily determine its size, not genetics at least for weight-bearing bones. If this weren’t the case, how would you explain that paralyzed children have much narrower tibias compared to normal individuals, possessing only 49% of the normal bone area [39]?
Okay, what about asymmetry? Is uneven growth possible? The answer is yes, but very rarely. Why? If some of you are aware of the effect of mechanical tension on bone growth, you might know that mechanical tension on one bone can lead to growth in the corresponding bone, even if it is not directly subjected to tension. Yes, it sounds unbelievable, but it is actually true.
In an astonishing study conducted on rats, scientists applied a direct impact to one tibia. They compared the impacted tibia with the opposite tibia that received no impact, and also with a control group that experienced no impact at all. The gene expression of BMP2 in the impacted tibia increased by about five and a half times. But the remarkable part is that in the non-impacted tibia of the same animal, BMP2 expression increased by roughly 3x of that amount. This is scientifically astonishing. The increase in the non-impacted bone was not minimal; it was almost half of the increase in the impacted bone. Scientists reported that the effect of trauma on bone is not just local but systemic, which is truly remarkable [40].
In reality, this is not the only study showing the same phenomenon. I do not know the exact scientific explanation for why mechanical tension on one bone leads to growth in the corresponding bone, but this is well-documented in the literature, even though there is no clear explanation for it.
For example, in another study on rats, the right humerus was mechanically loaded to see if the opposite, unloaded limb would also grow as an adaptive response, with comparisons made to a control group. What is remarkable is that the unloaded bone also grew, not just the loaded one. This effect is not limited to the humerus or to bone size. Scientists observed that not only the size but also the length of the bone increased in both the loaded and unloaded limbs compared to the control, whether for the leg, humerus, or femur. Both density and volume increased. In other words, any change that occurs in the loaded bone also occurs in the unloaded bone in the same animal [41,42,43].
Scientists explained the adaptation of the unloaded bone as being due to neural signals, and when neural inhibitors were used, the response in the unloaded bone almost disappeared. However, this may not be entirely accurate. It is reasonable to assume there is some neural communication between bones, so that when one bone is loaded, the nervous system sends a signal to stimulate growth in the opposite bone, whether in density, volume, or length.
At the same time, the same effect was observed in rats when scientists caused direct damage to the bone, not physiological loading. BMP2 activity increased by roughly 3xin the unloaded bone, about half of the increase in the damaged bone. They measured gene expression, not size or density, but a threefold increase in BMP2 usually means bone formation rate increased significantly.
It does not seem that the nervous system is the main mediator, because how could direct trauma activate neural signaling in the other bone? There is likely a deeper biological explanation perhaps a communication system between bones, or the body senses damage in one limb and builds the other to prepare. The exact mechanism is unclear, but the phenomenon is documented in rats and mice.
I cannot guarantee the same response in facial bones, but why not. What does all this mean? It means two things: first, the chance of asymmetry from bone smashing is low because there is a systemic response. Second, smart people have already thought about this: if the bone growth in rodeo riders were compared to another bone in the same body, would the growth be much greater? The logical answer is yes, because a systemic adaptive response has been proven. If, for example, the left limb continues to receive repeated impacts, the body will trigger a systemic response to increase the size of the corresponding bone. This has been documented in rats and mice. Why wouldn’t the same apply to humans if we have nearly the same biological mechanisms? So does this mean the massive bone growth seen in rodeo riders due to chronic periosteal inflammation could be much greater than we think if compared to a control limb from another person? The logical answer is yes
should also mention something important before ending this post. Some of you may already know about the ESWT method for increasing bone volume and how effective it is in animals and in speeding up fracture healing in humans. The scientific explanation of how this works is very similar to subperiosteal hematoma formation. A direct quote from studies: “ESWT can make old callus micro fracture through mechanical conduction, form subperiosteal hematoma, promote the release of bioactive factors.” What does this mean? ESWT can deliver strong waves that cause a subperiosteal hematoma and then release growth factors. Doesn’t this closely resemble the principle I explained? The only difference is that this method is well-documented to increase bone volume in both animals and humans [44].
Okay, after all this explanation and why asymmetry is unlikely and growth can be aesthetic, except if someone is mentally impaired and strikes randomly and imprecisely, can I guarantee perfectly aesthetic and symmetrical results? No. There is still a small chance of irregularity, even if limited. You can reduce this chance greatly by striking carefully and evenly. Bone grows where the subperiosteal hematoma forms. If you strike evenly, blood vessels rupture evenly, hematomas form evenly, and bone grows evenly. In the end, you control this process. Case reports show that random hematomas from careless strikes cause irregular bone growth, while proper, controlled hematomas lead to symmetrical and aesthetic growth, as seen in rodeo riders.
Conclusion:
The new theory suggests that bone smashing has a high potential to be effective through blood vessel rupture, subperiosteal hematoma formation, release of inflammatory factors, hypoxic environment, tensile forces, and electrical potential changes that stimulate gene expression for bone formation within the hematoma, leading to its calcification and transformation into new mature bone.
Tldr : fuck u niggers every thing is cope and its over
1. Physiology, Bone Remodeling
Paul Rowe; Adam Koller; Sandeep Sharma.
2. Ossification of subperiosteal hematoma: the potential of periosteal osteogenesis in cranioplasty
Yong Wang et al. J Craniofac Surg. 2013 Sep.
3. Subperiosteal Hematoma of the Orbit With Osteoneogenesis
Sina J. Sabet, MD; Kristin J. Tarbet, MD; Bradley N. Lemke, MD
4. Accelerated Bone Healing via Electrical Stimulation
Jianfeng Sun et al. Adv Sci (Weinh). 2025 Jun.
5. The effects and underlying mechanism of extracorporeal shockwave therapy on fracture healing
Fuxian Lv 1, Zhenlan Li 1, Yuling Jing 1, Liyuan Sun 1, Zhiwei Li 1, Haoyang Duan 1,*
6. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair
by Qiuyue Qin, Yiping Liu, Zhen Yang, Maierhaba Aimaijiang, Rui Ma, Yixin Yang, Yidi Zhang * and Yanmin Zhou
7. Experimental study of free periosteal autograft. Animals age and periosteal osteogenesis
W G Li et al. Chin Med J (Engl). 1989 May
8. Periosteal Skeletal Stem Cells and Their Response to Bone Injury
Nian Zhang 1, Liru Hu 1, Zhiwei Cao 1, Xian Liu 1, Jian Pan 1,*
9. The Role of Piezoelectric Materials in Bone Remodeling and Repair: Mechanisms and Applications
10. The Role of the Periosteum in Bone Formation From Adolescence to Old Age
11. Differential survival among individuals with active and healed
periosteal new bone formation
Sharon N. DeWitte∗
Department of Anthropology, University of South Carolina, Columbia, SC 29208, United States
12. Periosteum: An imaging review”
Carlos Henrique Maia Ferreira Alencar a,*, Cláudio Régis Sampaio Silveira a, Matheus Martins Cavalcante a, Clarissa Gadelha Maia Vieira b, Manoel Joaquim Diógenes Teixeira c, Francisco Andrade Neto d, Armando de Abreu e, Avneesh Chhabra f
12. Periosteal reaction with normal-appearing underlying bone: a child abuse mimicker
Nipa Ved et al. Emerg Radiol. 2002 Nov
13. Management of peripheral pain generators in fibromyalgia
2002, Rheumatic Disease Clinics of North America
14. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis
Takahiro Kanno et al. J Oral Maxillofac Surg. 2005 Apr.
15. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, M
16. Modulation of the Inflammatory Response and Bone Healing
Masahiro MaruyamaMasahiro Maruyama1Claire RheeClaire Rhee1Takeshi UtsunomiyaTakeshi Utsunomiya1Ning ZhangNing Zhang1Masaya UenoMasaya Ueno1Zhenyu YaoZhenyu Yao1Stuart B. Goodman,
Stuart B. Goodman1,2*
17. Cellular Biology of Fracture Healing
Chelsea S Bahney 1, Robert L Zondervan 2,3, Patrick Allison 2, Alekos Theologis 1, Jason W Ashley 4, Jaimo Ahn 4, Theodore Miclau 1, Ralph S Marcucio 1, Kurt D Hankenson
18. Fracture Healing Overview
Jonathon R. Sheen; Ahmed Mabrouk; Vishnu V. Garla.
Author Information and Affiliations
Last Update: April 8, 2023.
Go
19. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, MD
20. Traumatic subperiosteal pseudoaneurysm: rare cause of subperiosteal hematoma
Hyuk Joong Choi et al. Am J Emerg Med. 2009 Nov.
21. Molecular profiling of a simple rat model of open tibial fractures with hematoma and periosteum disruption
Jose Rafael Villafan-Bernal et al. Exp Ther Med. 2016 Nov.
22. Skeletal Trauma Increases the Expression of Specific Mesenchymal Stem Cell Markers and Bone Morphogenetic
Protein-2 in a Systemic Manner
Richard Marsell1, Brandon Steen1, Manish Bais 1, Douglas P. Mortlock2 Louis C. Gerstenfeld1, Thomas A. Einhorn1
1Department of Orthopaedic Surgery, Boston University Med
23. OSSIFIED SUBPERIOSTEAL HEMATOMA
OBSERVATIONS ON THREE INSTANCES INCLUDING ONE IN AN EXTINCT RUMINANT, THE IRISH GIANT DEER
24. Skin Cell Proliferation Stimulated by Microneedles
25. Bony hypertrophy of the forearm in bareback rodeo athletes
Christian Douthit et al. SAGE Open Med. 2022.
26. Chronic Hypertrophy of the Ulna in the Professional Rodeo Cowboy BRUCE F. CLAUSSEN, M.D
27. Myositis ossificans circumscripta of the thigh: A pediatric case report
Chaymae Faraj a,⁎, Sara Essetti a, Yahya El Harras a, Najlae Lrhorfi a, Nidal Mrani Alaoui b, Mohamed Anouar Dendane b, Abdelouahed Amrani b, Sidi Zouhair El Fellous El Alami b, Tarik El Madhi b, Nazik Allali a, Siham El Haddad a, Latifa Chat a
28. Case Report: Ossified Subperiosteal Hematoma in the Humerus of a Lacrosse Player
29. Subperiosteal Hematoma of the Ankle
S H Hui 1, T H Lui
30. 852 Traumatic Ossifying Periostitis of the Ulna Masquerading as a Malignancy in a Football Player A Case Report and Literature Review William G. Ward, Sr.,*† MD, Jon K. Sekiya,*
31. A Case Report & Literature Review
Florid Reactive Periostitis of the Hand
Richard Vinglas, MD, and Stephen B. Schnall, M
32. Atypical Presentation of Non-Ossifying Fibroma in a Professional Muay Thai Boxer: A Case Report and a Narrative Review of the Literature
Marco Pes 1,✉, Alessio Pulino 1, Umberto Cardinale 1, Francesco Pisanu 1, Andrea Fabio Manunta
33. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, MD
34. Post-Traumatic Peripheral Giant Osteoma in the Frontal Bone
Seong Hwan Kim 1, Dong Seob Lim 1, Do Hun Lee 1, Kyung Pil Kim 1, Jae Ha Hwang 1,✉, Kwang Seog Kim 1, Sam Yong Lee 1
35. Ossification of a Post-Traumatic Low Frontal Subcutaneous
Hematoma Treated via Trans-Eyebrow Approach: A
Clinical Case
36. Subperiosteal Hematoma of the Orbit With Osteoneogenesis
Sina J. Sabet, MD; Kristin J. Tarbet, MD; Bradley N. Lemke, MD
37. Mandibular traumatic peripheral osteoma: a case report
Ruggero Rodriguez y Baena, MD, DDS," Silvana Rizzo, MD, DDS," Giacomo Fiandrino, MD,' Saturnino Lupi, DDS," and Silvestre Galioto, MD,* Pavia, Italy UNIVERSITY OF PAVIA
38. Experimental study of free periosteal autograft. Animals age and periosteal osteogenesis
W G Li et al. Chin Med J (Engl). 1989 May.
39. Mechanical signaling for bone modeling and remodeling
Alexander G Robling et al. Crit Rev Eukaryot Gene Expr. 2009
40. Skeletal Trauma Increases the Expression of Specific Mesenchymal Stem Cell Markers and Bone Morphogenetic
Protein-2 in a Systemic Manner
Richard Marsell1, Brandon Steen1, Manish Bais 1, Douglas P. Mortlock2 Louis C. Gerstenfeld1, Thomas A. Einhorn1
1Department of Orthopaedic Surgery, Boston University Medical Center, 715 Albany Street, R-205, Boston, MA 02118
2Department of Molecular Physiology and Biophysics, Center for Human Genetics Research, Vanderbilt University School of Medicine, Nashville, TN
41. Functional Adaptation to Loading of a Single Bone Is Neuronally Regulated and Involves Multiple Bones†, ‡
Susannah J Sample 1, Mary Behan 1,2, Lesley Smith 3, William E Oldenhoff 1, Mark D Markel 1,4, Vicki L Kalscheur 1, Zhengling Hao 1, Vjekoslav Miletic 5, Peter Muir 1,3
42. Lengthening of mouse hindlimbs with joint loading
Ping Zhang et al. J Bone Miner Metab. 2010 May.
43. Elbow loading promotes longitudinal bone growth of the ulna and the humerus
Ping Zhang et al. J Bone Miner Metab. 2012 Jan.
44. The effects and underlying mechanism of extracorporeal shockwave therapy on fracture healing
Fuxian Lv 1, Zhenlan Li 1, Yuling Jing 1, Liyuan Sun 1, Zhiwei Li 1, Haoyang Duan
Bone smashing is one of the most popular methods on this forum and in fact not just on this forum but in the looksmaxing community in general.The idea behind it is that hitting the bone will create mechanical stress on it which will lead to an increase in its size and people usually refer to Wolff’s law to prove this point.But this is completely illogical.First Wolff never mentioned that bone size increases due to mechanical stress all he mentioned was bone density.Second Wolff stated that physiological mechanical stress resulting from daily activities is what causes bone adaptation not random hits on the bone [1].So it’s clear that interpreting the effectiveness of bone smashing based on a loosely defined law created by a German surgeon more than 120 years ago and misinterpreting it is not proof at all of the effectiveness of hitting your facial bones to increase their size.Does that mean bone smashing is ineffective?Well not exactly.Bone smashing might actually be the only scientifically proven method to increase bone size but certainly not based on a random law written by a German scientist and misinterpreted by kids.There’s something much deeper than that and it’s called “subperiosteal hematoma ossification.”You may have already heard this term before from my previous post but to be honest the explanation itself was one thing while the organization of information and the way it was presented were somewhat off even if the idea I wanted to convey was somewhat clear
Note before starting the explanation everything I say is 100% based on science all sources have already been included by the time you’re reading this post)Okay what is a subperiosteal hematoma?Simply it’s a phenomenon that occurs due to blood leaking between the periosteum and the bone layer because of a rupture in the blood vessels which leads to the periosteum being lifted off the bone forming a shape that resembles a “mountain plateau.”There are several causes that can lead to the formation of a subperiosteal hematoma but the most common and scientifically agreed upon cause is direct hits or trauma to the bone.Why?Because it leads to the rupture of blood vessels then the leakage of blood between the periosteum and the bone which results in the lifting of the periosteum layer off the bone (See figure 1 to understand)

(Figure 1 you can see a subperiosteal hematoma and a partial elevation or separation of the periosteum from the bone)
Okay I think the concept of subperiosteal hematoma is already clear but what is its ossification?How does it ossify?And what is the scientific explanation for that?Well there are four hypotheses that explain it logically and I will explain them (of course everything is scientifically based) then I will show documented evidence of massive bone hypertrophy that occurs due to the ossification of a subperiosteal hematoma
The first theory the inflammatory environment (the most important factor):When a subperiosteal hematoma occurs as a result of an injury or a direct blow to the bone the blood vessels connecting the periosteum and the bone rupture leading to blood accumulation and the spread of red blood cells.After some time these cells coagulate and cause the release of a group of inflammatory factors from the blood components such as platelets macrophages and other immune cells.These factors include cytokines such as IL-1β IL-6 TNF-α as well as chemokines like MCP-1 and growth factors such as TGF-β VEGF BMPs and cox2.These factors create a favorable environment for stimulating bone formation through several biological mechanisms.First the inflammatory cytokines help attract mesenchymal stem cells (MSCs) from the bone marrow and the inner layer of the periosteum which is very rich in MSCs to the injury site.These stem cells migrate toward the affected area due to chemokine signals and are activated by cytokines and inflammatory mediators like IL-6 and TGF-β.Upon arrival the stem cells begin differentiating into osteoblasts thanks to stimulation by growth factors such as BMPs and TGF-β leading to the secretion of the initial bone matrix composed of collagen and calcium salts.At the cellular level these factors activate signaling pathways such as SMAD MAPK and JAK/STAT.The importance of inflammatory factors can be easily proven by the fact that when inflammatory factors are removed there is a severe delay in bone healing
The second theory change in electrical potential:When a subperiosteal hematoma occurs due to an injury or a fracture blood accumulates between the periosteum and the bone leading to mechanical pressure and electrical changes that affect bone cells and the healing process.If anyone here knows what microneedling for the skin is you probably know that the main reason for its effectiveness is not what people usually think but actually because of changes in electrical potential which makes the body believe that the skin has been torn or injured when in reality nothing happened except a change in electrical potential so the body releases growth factors to heal it.The same concept likely applies to bone.Bone has piezoelectric properties meaning it generates electrical signals when subjected to stress or strain.When blood accumulates under the periosteum it changes the mechanical stress pattern on the bone generating a local electrical potential in the affected area.This electrical potential acts as a signal that stimulates osteocytes and mesenchymal stem cells (MSCs) in the area.The stem cells respond to these electrical signals by migrating to the injury site via integrin receptors on their surface and differentiating into osteoblasts.Once these cells reach the site they begin secreting bone matrix made of collagen and calcium salts contributing to the formation of new bone.Furthermore the change in electrical stress activates signaling pathways such as the calcium pathway and MAPK leading to the opening of calcium channels in bone cells.Simply put this theory is based on the idea that the change in electrical potential tricks your body into believing that a fracture has occurred in the bone when in reality nothing has happened to it
The third theory severe oxygen deprivation:When the bone is exposed to a direct impact the blood vessels rupture leading to blood clotting which creates a highly hypoxic environment.You might think this is a bad thing but in reality it’s the opposite.Recent research shows that hypoxia plays a crucial and essential role in bone formation.The rupture of blood vessels cuts off the oxygen supply to the bone which acts as a trigger for the body to increase the expression of a gene called HIF-1α (Hypoxia Inducible Factor-1 alpha).This protein or gene is very important for osteogenic differentiation through multiple pathways.First by increasing the expression of VEGF second by enhancing the activity of the Wnt/β-catenin signaling pathway and also by upregulating BMPs.Moreover it inhibits the activity of Twist2 which leads to an increase in the gene expression of Runx2 and Osterix.Simply the hypoxic environment is ideal for the differentiation of stem cells attracted from the inner layer of the periosteum by inflammatory factors as it drives them to differentiate into osteogenic cells and form a mature and complete bone matrix at the injury site (the site of the hematoma)
The fourth theory tensile force caused by the hematoma:This hypothesis may be the most logical one since many scientists have stated that it is the most reasonable explanation for the ossification of a subperiosteal hematoma.This theory suggests that the hematoma lifts the periosteum away from the bone layer which activates the MSCs located in the inner layer of the periosteum (the cambium layer) through their mechanoreceptors such as primary cilia integrins and purinergic receptors.Researchers supported this hypothesis based on an in vitro study where stem cells extracted from the inner layer of the periosteum were subjected to tensile force (similar to what cells might experience during a hematoma) and this indeed led to an increased gene expression of Runx2 Osterix and BMP which are crucial genes and proteins responsible for driving MSCs to differentiate into osteogenic cells
In summary, hitting the bone causes the blood vessels to rupture which creates an inflammatory environment, hypoxia, changes in electrical potential, and tensile stress on stem cells, leading to their transformation into a new bone layer (often this transformation occurs directly without a cartilage template since the periosteum itself is not damaged).When repeated hits are applied continuously over months or years, a condition called chronic peripheral periostitis occurs, resulting in the formation of a prominent bony mass at the site of injury.
(Note, as I mentioned earlier everything is scientifically based, and for the scientific sources they are [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23])
Okay, is there clinical evidence proving that a subperiosteal hematoma can ossify and become bone?The answer is yes, in fact there are hundreds or even thousands of studies, evidence, and case reports supporting this.I will now present some of them and show evidence proving that the mechanism I mentioned applies to any bone in the body, and I will definitely provide supporting proof for that.
Many of you may already know about bareback rodeo, a sport that requires the rider to have their forearm directly strike the hip bone. Here is a literal excerpt from the study describing what happens: “On observing a contestant who is riding, the riding arm is noted to contact the anterior iliac crest and the heavily resined chaps (Fig. 3). The event requires strength, timing, balance, and courage. Ideally, the forearm should be tucked against the pelvis and chaps. Failure to maintain this position results in a hammering action of the forearm against the pelvis and chaps.“You don’t have to be particularly smart to understand this literally; the scientists stated that the hypertrophy that occurs is the result of direct impact or a “hammering action.”If you’re wondering about the extent of the bone growth, to be frank there was massive hypertrophy, although it varied among participants (I will explain the reasons for this variation).In one sample, the diameter of the ulna increased by 92% and the bone’s cross-sectional area by 262%, which are extremely large biological increases, especially considering participation in this sport is prohibited before age 18.Some might argue that this hypertrophy is due to muscle tension rather than direct impact on the bone, but this is incorrect for several reasons.First, many sports apply intense torsion forces to bones but do not cause even a 10% increase as observed in rodeo riders.Second, the hypertrophy occurs exclusively at the area where the ulna contacts the hip.Third, the hypertrophy was observed only in riders who did not use a saddle; the saddle acts as a barrier preventing friction and impact with the hip bone, and its absence allows direct friction and impact.Fourth, the variation in hypertrophy among participants is likely because some wore protective gear to prevent friction while others did not (see figure 2 to view the bone hypertrophy) [25].

(Figure 2, an image showing bone hypertrophy, please note that the increase in diameter here was approximately 62%, but the largest increase reached 92%)
In another study with the same objective as the first but older, we can say that they found exactly the same results massive bone hypertrophy occurred in bareback rodeo riders. Fortunately, this time the supervising researcher fully agrees with my scientific explanation for this hypertrophy. I will quote directly from him: “The hypertrophy is considered by the author to be a chronic circumferential periostitis secondary to trauma.“It seems the author completely agrees with me, explaining the hypertrophy as resulting from direct trauma to the bone (the trauma from the ulna hitting the pelvis), which leads to chronic peripheral periostitis and subsequently bone hypertrophy.This study strongly supports my theory, because how else would you explain massive bone growth resulting from periosteal inflammation (as we mentioned earlier, through hematoma formation, red blood cell coagulation, and release of inflammatory factors)? Continuing in this state eventually leads to enormous bone hypertrophy.An important point is that a rodeo round actually lasts only 8 seconds, which debunks the argument that you need to spend hours daily to do bone smashing. If that were true, how do you explain the bone hypertrophy in rodeo riders who only do very limited rounds per day?Not to mention that the bone isn’t in constant impact throughout the round, probably only 2–3 seconds in total (see figure 3 to view the hypertrophy) [26]

(Figure 3, bone hypertrophy resulting from practicing rodeo)
The evidence supporting my theory is not limited to these studies. In fact, there is much more. For example, a 15-year-old soccer player experienced a direct injury to his knee. What happened next? A subperiosteal hematoma formed, followed by bone ossification and the appearance of a clear bony mass on X-ray [27]. There are many similar case reports. For instance, in a lacrosse player, a sport that involves direct contact with opponents, the athlete reported pain in an area left unprotected. That area continued to experience direct hits, bleeding, and eventually bone hypertrophy. Scientists described this exactly as it happened, and indeed this was confirmed on X-ray [28].
In another case, a person fell on their ankle and was injured. After a while, a subperiosteal hematoma formed, which then ossified to become bone (we have already explained how a hematoma ossifies). I am not making this up; this is literally what the study and researchers reported [29]. In another case, a soccer defender’s humerus continued to experience direct impact during defense, leading to a subperiosteal hematoma, then ossification, and finally transformation into bone. The scientists reported that this hypertrophy was mainly due to repeated microtrauma to the bone [30].
In another individual case, a woman hit her finger on a cabinet, leading to a hematoma, which then ossified into new bone [31]. In another case, a Muay Thai boxer experienced massive bone hypertrophy clearly visible on X-ray, which scientists attributed to repeated microtrauma [32]. In another individual case, a child fell on their pelvis while skiing, and radiographs showed very dense bone hypertrophy at the injury site due to a subperiosteal hematoma (see figure 4 to view the hypertrophy) [33].

(Figure 4, an image showing a dense bony mass resulting from the periosteum being lifted off the bone layer and ossification of the hematoma)
So, it seems I have already presented many studies and case reports documenting the phenomenon I discussed, which is blood vessel rupture, followed by subperiosteal hematoma formation, and then ossification. I have also conclusively shown that this mechanism applies to all bones in the body, such as the hip, femur, tibia, hand, and fingers. Please note that there are hundreds, if not thousands, of case reports documenting the same process repeatedly. But what about the skull bones? Are there any cases documenting subperiosteal hematoma formation and subsequent ossification? The answer is yes. In fact, case reports usually show severe bone hypertrophy at the injury site, possibly because the periosteum’s attachment to the skull is looser compared to other bones, making subperiosteal hematoma formation easier.
For example, in a case of a 35-year-old woman who experienced a severe blow to her frontal bone, what happened? As expected, a subperiosteal hematoma formed, then ossified into a large bony mass. Interestingly, the growth continued even after several years. The exact scientific explanation for the continued bone growth after years from the injury is unclear, but it may be an exaggerated protective response by the body (see figure 5) [34].

(Figure 5: a prominent bony mass at the injury site formed years after ossification of the subperiosteal hematoma)
In another case, a young child experienced a direct blow to the orbital bone while watching a match. After some time, the hematoma ossified, forming a very large bony mass at the injury site after 11 months. Scientists reported that the hypertrophy was due to the release of inflammatory factors and the tensile force applied to the periosteum [35]. In another case, a child sustained an injury to the side of the supraorbital area, and similarly, bone hypertrophy was observed at the injury site [36]. Not only that, in another case, a person received a blow to the lower jaw while playing soccer, and after some time, bone formation appeared at the injury site (see figure 6) [37].

(Figure 6: a prominent bony mass in the lower jaw resulting from a direct injury to the mandible during a soccer match)
Discussion:
It seems that, without a doubt, the theory is correct. This has been documented in all cases, and most scientists usually explain the result by two factors: first, the release of inflammatory factors due to the coagulation of red blood cells; second, the tensile force applied by the hematoma on the MSCs in the periosteum. There are hundreds, if not thousands, of pieces of evidence documenting the same phenomenon.
It is also important to mention something crucial: the younger the person, the faster the bone growth. Why? This is the same reason why fractures heal much faster in children than in adults, due to the higher activity of stem cells in the inner layer of the periosteum. This means that anyone still in puberty has a golden opportunity to exploit a subperiosteal hematoma to transform it into bone.
Okay, let’s be realistic. Will the bone growth be aesthetic? The answer depends on the person. Bone growth won’t happen magically or randomly; it will simply occur where the hematoma forms, or where the blood vessels rupture. So why does bone formation often appear random on X-rays? The answer is simple: the hematoma occurred randomly because the impact was random.
Remember, the body doesn’t build bone to make you more aesthetic. It simply builds bone for protection. So regardless of whether the bone grows aesthetically or not, you are in control. You determine when and where the blood vessels rupture. I can easily prove that the hematoma can be aesthetic. When people here perform bone smashing, they often report that the hematoma that forms is aesthetic.
Logically, the blood will ossify only at the injury site. Nothing magical grows randomly unless the hematoma itself is random. I can also prove this by looking at rodeo riders, where bone hypertrophy is natural and not random. Why? Simply because the bone grows only at the spot receiving the impact. If growth were truly random, why do we observe that bone growth in rodeo riders is natural and even aesthetic? Anyone who thinks bone growth cannot be aesthetic must answer all the questions I just posed.
Okay, this theory refutes any argument against bone smashing. For example, it refutes the claim that you must be young for it to work, which is not true. For instance, stem cells in the periosteum of rabbits are very active at all life stages and have the ability to form bone at any age [38]. But what about humans? The answer is yes. If it weren’t, how would you explain bone healing after fractures in elderly people? The only explanation is that periosteal stem cells remain active and are reactivated during fracture, just as shown in the rabbit study. Yes, of course, the younger you are, the more active they are there is no doubt about that.
This theory also refutes the idea that direct bone impact won’t stimulate bone growth, and that you need physiological force rather than direct strikes to the bone. The theory is based on the fact that blood vessels must be ruptured for blood to escape, and direct impact on the bone is very effective at this. It also refutes the notion that bone increases in size from mechanical tension alone, because there is plenty of evidence showing that hematoma ossification has a high potential for ossification (the younger you are, the higher the chance). In reality, mechanical tension on a specific bone may primarily determine its size, not genetics at least for weight-bearing bones. If this weren’t the case, how would you explain that paralyzed children have much narrower tibias compared to normal individuals, possessing only 49% of the normal bone area [39]?
Okay, what about asymmetry? Is uneven growth possible? The answer is yes, but very rarely. Why? If some of you are aware of the effect of mechanical tension on bone growth, you might know that mechanical tension on one bone can lead to growth in the corresponding bone, even if it is not directly subjected to tension. Yes, it sounds unbelievable, but it is actually true.
In an astonishing study conducted on rats, scientists applied a direct impact to one tibia. They compared the impacted tibia with the opposite tibia that received no impact, and also with a control group that experienced no impact at all. The gene expression of BMP2 in the impacted tibia increased by about five and a half times. But the remarkable part is that in the non-impacted tibia of the same animal, BMP2 expression increased by roughly 3x of that amount. This is scientifically astonishing. The increase in the non-impacted bone was not minimal; it was almost half of the increase in the impacted bone. Scientists reported that the effect of trauma on bone is not just local but systemic, which is truly remarkable [40].
In reality, this is not the only study showing the same phenomenon. I do not know the exact scientific explanation for why mechanical tension on one bone leads to growth in the corresponding bone, but this is well-documented in the literature, even though there is no clear explanation for it.
For example, in another study on rats, the right humerus was mechanically loaded to see if the opposite, unloaded limb would also grow as an adaptive response, with comparisons made to a control group. What is remarkable is that the unloaded bone also grew, not just the loaded one. This effect is not limited to the humerus or to bone size. Scientists observed that not only the size but also the length of the bone increased in both the loaded and unloaded limbs compared to the control, whether for the leg, humerus, or femur. Both density and volume increased. In other words, any change that occurs in the loaded bone also occurs in the unloaded bone in the same animal [41,42,43].
Scientists explained the adaptation of the unloaded bone as being due to neural signals, and when neural inhibitors were used, the response in the unloaded bone almost disappeared. However, this may not be entirely accurate. It is reasonable to assume there is some neural communication between bones, so that when one bone is loaded, the nervous system sends a signal to stimulate growth in the opposite bone, whether in density, volume, or length.
At the same time, the same effect was observed in rats when scientists caused direct damage to the bone, not physiological loading. BMP2 activity increased by roughly 3xin the unloaded bone, about half of the increase in the damaged bone. They measured gene expression, not size or density, but a threefold increase in BMP2 usually means bone formation rate increased significantly.
It does not seem that the nervous system is the main mediator, because how could direct trauma activate neural signaling in the other bone? There is likely a deeper biological explanation perhaps a communication system between bones, or the body senses damage in one limb and builds the other to prepare. The exact mechanism is unclear, but the phenomenon is documented in rats and mice.
I cannot guarantee the same response in facial bones, but why not. What does all this mean? It means two things: first, the chance of asymmetry from bone smashing is low because there is a systemic response. Second, smart people have already thought about this: if the bone growth in rodeo riders were compared to another bone in the same body, would the growth be much greater? The logical answer is yes, because a systemic adaptive response has been proven. If, for example, the left limb continues to receive repeated impacts, the body will trigger a systemic response to increase the size of the corresponding bone. This has been documented in rats and mice. Why wouldn’t the same apply to humans if we have nearly the same biological mechanisms? So does this mean the massive bone growth seen in rodeo riders due to chronic periosteal inflammation could be much greater than we think if compared to a control limb from another person? The logical answer is yes
should also mention something important before ending this post. Some of you may already know about the ESWT method for increasing bone volume and how effective it is in animals and in speeding up fracture healing in humans. The scientific explanation of how this works is very similar to subperiosteal hematoma formation. A direct quote from studies: “ESWT can make old callus micro fracture through mechanical conduction, form subperiosteal hematoma, promote the release of bioactive factors.” What does this mean? ESWT can deliver strong waves that cause a subperiosteal hematoma and then release growth factors. Doesn’t this closely resemble the principle I explained? The only difference is that this method is well-documented to increase bone volume in both animals and humans [44].
Okay, after all this explanation and why asymmetry is unlikely and growth can be aesthetic, except if someone is mentally impaired and strikes randomly and imprecisely, can I guarantee perfectly aesthetic and symmetrical results? No. There is still a small chance of irregularity, even if limited. You can reduce this chance greatly by striking carefully and evenly. Bone grows where the subperiosteal hematoma forms. If you strike evenly, blood vessels rupture evenly, hematomas form evenly, and bone grows evenly. In the end, you control this process. Case reports show that random hematomas from careless strikes cause irregular bone growth, while proper, controlled hematomas lead to symmetrical and aesthetic growth, as seen in rodeo riders.
Conclusion:
The new theory suggests that bone smashing has a high potential to be effective through blood vessel rupture, subperiosteal hematoma formation, release of inflammatory factors, hypoxic environment, tensile forces, and electrical potential changes that stimulate gene expression for bone formation within the hematoma, leading to its calcification and transformation into new mature bone.
Tldr : fuck u niggers every thing is cope and its over
1. Physiology, Bone Remodeling
Paul Rowe; Adam Koller; Sandeep Sharma.
2. Ossification of subperiosteal hematoma: the potential of periosteal osteogenesis in cranioplasty
Yong Wang et al. J Craniofac Surg. 2013 Sep.
3. Subperiosteal Hematoma of the Orbit With Osteoneogenesis
Sina J. Sabet, MD; Kristin J. Tarbet, MD; Bradley N. Lemke, MD
4. Accelerated Bone Healing via Electrical Stimulation
Jianfeng Sun et al. Adv Sci (Weinh). 2025 Jun.
5. The effects and underlying mechanism of extracorporeal shockwave therapy on fracture healing
Fuxian Lv 1, Zhenlan Li 1, Yuling Jing 1, Liyuan Sun 1, Zhiwei Li 1, Haoyang Duan 1,*
6. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair
by Qiuyue Qin, Yiping Liu, Zhen Yang, Maierhaba Aimaijiang, Rui Ma, Yixin Yang, Yidi Zhang * and Yanmin Zhou
7. Experimental study of free periosteal autograft. Animals age and periosteal osteogenesis
W G Li et al. Chin Med J (Engl). 1989 May
8. Periosteal Skeletal Stem Cells and Their Response to Bone Injury
Nian Zhang 1, Liru Hu 1, Zhiwei Cao 1, Xian Liu 1, Jian Pan 1,*
9. The Role of Piezoelectric Materials in Bone Remodeling and Repair: Mechanisms and Applications
10. The Role of the Periosteum in Bone Formation From Adolescence to Old Age
11. Differential survival among individuals with active and healed
periosteal new bone formation
Sharon N. DeWitte∗
Department of Anthropology, University of South Carolina, Columbia, SC 29208, United States
12. Periosteum: An imaging review”
Carlos Henrique Maia Ferreira Alencar a,*, Cláudio Régis Sampaio Silveira a, Matheus Martins Cavalcante a, Clarissa Gadelha Maia Vieira b, Manoel Joaquim Diógenes Teixeira c, Francisco Andrade Neto d, Armando de Abreu e, Avneesh Chhabra f
12. Periosteal reaction with normal-appearing underlying bone: a child abuse mimicker
Nipa Ved et al. Emerg Radiol. 2002 Nov
13. Management of peripheral pain generators in fibromyalgia
2002, Rheumatic Disease Clinics of North America
14. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis
Takahiro Kanno et al. J Oral Maxillofac Surg. 2005 Apr.
15. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, M
16. Modulation of the Inflammatory Response and Bone Healing
Masahiro MaruyamaMasahiro Maruyama1Claire RheeClaire Rhee1Takeshi UtsunomiyaTakeshi Utsunomiya1Ning ZhangNing Zhang1Masaya UenoMasaya Ueno1Zhenyu YaoZhenyu Yao1Stuart B. Goodman,
Stuart B. Goodman1,2*
17. Cellular Biology of Fracture Healing
Chelsea S Bahney 1, Robert L Zondervan 2,3, Patrick Allison 2, Alekos Theologis 1, Jason W Ashley 4, Jaimo Ahn 4, Theodore Miclau 1, Ralph S Marcucio 1, Kurt D Hankenson
18. Fracture Healing Overview
Jonathon R. Sheen; Ahmed Mabrouk; Vishnu V. Garla.
Author Information and Affiliations
Last Update: April 8, 2023.
Go
19. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, MD
20. Traumatic subperiosteal pseudoaneurysm: rare cause of subperiosteal hematoma
Hyuk Joong Choi et al. Am J Emerg Med. 2009 Nov.
21. Molecular profiling of a simple rat model of open tibial fractures with hematoma and periosteum disruption
Jose Rafael Villafan-Bernal et al. Exp Ther Med. 2016 Nov.
22. Skeletal Trauma Increases the Expression of Specific Mesenchymal Stem Cell Markers and Bone Morphogenetic
Protein-2 in a Systemic Manner
Richard Marsell1, Brandon Steen1, Manish Bais 1, Douglas P. Mortlock2 Louis C. Gerstenfeld1, Thomas A. Einhorn1
1Department of Orthopaedic Surgery, Boston University Med
23. OSSIFIED SUBPERIOSTEAL HEMATOMA
OBSERVATIONS ON THREE INSTANCES INCLUDING ONE IN AN EXTINCT RUMINANT, THE IRISH GIANT DEER
24. Skin Cell Proliferation Stimulated by Microneedles
25. Bony hypertrophy of the forearm in bareback rodeo athletes
Christian Douthit et al. SAGE Open Med. 2022.
26. Chronic Hypertrophy of the Ulna in the Professional Rodeo Cowboy BRUCE F. CLAUSSEN, M.D
27. Myositis ossificans circumscripta of the thigh: A pediatric case report
Chaymae Faraj a,⁎, Sara Essetti a, Yahya El Harras a, Najlae Lrhorfi a, Nidal Mrani Alaoui b, Mohamed Anouar Dendane b, Abdelouahed Amrani b, Sidi Zouhair El Fellous El Alami b, Tarik El Madhi b, Nazik Allali a, Siham El Haddad a, Latifa Chat a
28. Case Report: Ossified Subperiosteal Hematoma in the Humerus of a Lacrosse Player
29. Subperiosteal Hematoma of the Ankle
S H Hui 1, T H Lui
30. 852 Traumatic Ossifying Periostitis of the Ulna Masquerading as a Malignancy in a Football Player A Case Report and Literature Review William G. Ward, Sr.,*† MD, Jon K. Sekiya,*
31. A Case Report & Literature Review
Florid Reactive Periostitis of the Hand
Richard Vinglas, MD, and Stephen B. Schnall, M
32. Atypical Presentation of Non-Ossifying Fibroma in a Professional Muay Thai Boxer: A Case Report and a Narrative Review of the Literature
Marco Pes 1,✉, Alessio Pulino 1, Umberto Cardinale 1, Francesco Pisanu 1, Andrea Fabio Manunta
33. Iliac Subperiosteal Hematoma with Ossification in a 15-Year-Old Boy
Sun Hwa Lee, MD ∙ Seong Jong Yun, MD
34. Post-Traumatic Peripheral Giant Osteoma in the Frontal Bone
Seong Hwan Kim 1, Dong Seob Lim 1, Do Hun Lee 1, Kyung Pil Kim 1, Jae Ha Hwang 1,✉, Kwang Seog Kim 1, Sam Yong Lee 1
35. Ossification of a Post-Traumatic Low Frontal Subcutaneous
Hematoma Treated via Trans-Eyebrow Approach: A
Clinical Case
36. Subperiosteal Hematoma of the Orbit With Osteoneogenesis
Sina J. Sabet, MD; Kristin J. Tarbet, MD; Bradley N. Lemke, MD
37. Mandibular traumatic peripheral osteoma: a case report
Ruggero Rodriguez y Baena, MD, DDS," Silvana Rizzo, MD, DDS," Giacomo Fiandrino, MD,' Saturnino Lupi, DDS," and Silvestre Galioto, MD,* Pavia, Italy UNIVERSITY OF PAVIA
38. Experimental study of free periosteal autograft. Animals age and periosteal osteogenesis
W G Li et al. Chin Med J (Engl). 1989 May.
39. Mechanical signaling for bone modeling and remodeling
Alexander G Robling et al. Crit Rev Eukaryot Gene Expr. 2009
40. Skeletal Trauma Increases the Expression of Specific Mesenchymal Stem Cell Markers and Bone Morphogenetic
Protein-2 in a Systemic Manner
Richard Marsell1, Brandon Steen1, Manish Bais 1, Douglas P. Mortlock2 Louis C. Gerstenfeld1, Thomas A. Einhorn1
1Department of Orthopaedic Surgery, Boston University Medical Center, 715 Albany Street, R-205, Boston, MA 02118
2Department of Molecular Physiology and Biophysics, Center for Human Genetics Research, Vanderbilt University School of Medicine, Nashville, TN
41. Functional Adaptation to Loading of a Single Bone Is Neuronally Regulated and Involves Multiple Bones†, ‡
Susannah J Sample 1, Mary Behan 1,2, Lesley Smith 3, William E Oldenhoff 1, Mark D Markel 1,4, Vicki L Kalscheur 1, Zhengling Hao 1, Vjekoslav Miletic 5, Peter Muir 1,3
42. Lengthening of mouse hindlimbs with joint loading
Ping Zhang et al. J Bone Miner Metab. 2010 May.
43. Elbow loading promotes longitudinal bone growth of the ulna and the humerus
Ping Zhang et al. J Bone Miner Metab. 2012 Jan.
44. The effects and underlying mechanism of extracorporeal shockwave therapy on fracture healing
Fuxian Lv 1, Zhenlan Li 1, Yuling Jing 1, Liyuan Sun 1, Zhiwei Li 1, Haoyang Duan
Last edited: