LVZZO
̿̿ ̿̿ ̿̿ ̿'̿'\̵͇̿̿\з= ( ▀ ͜͞ʖ▀) =ε/̵͇̿̿/’̿’̿ ̿ ̿̿
- Joined
- Apr 23, 2023
- Posts
- 2,462
- Reputation
- 3,035
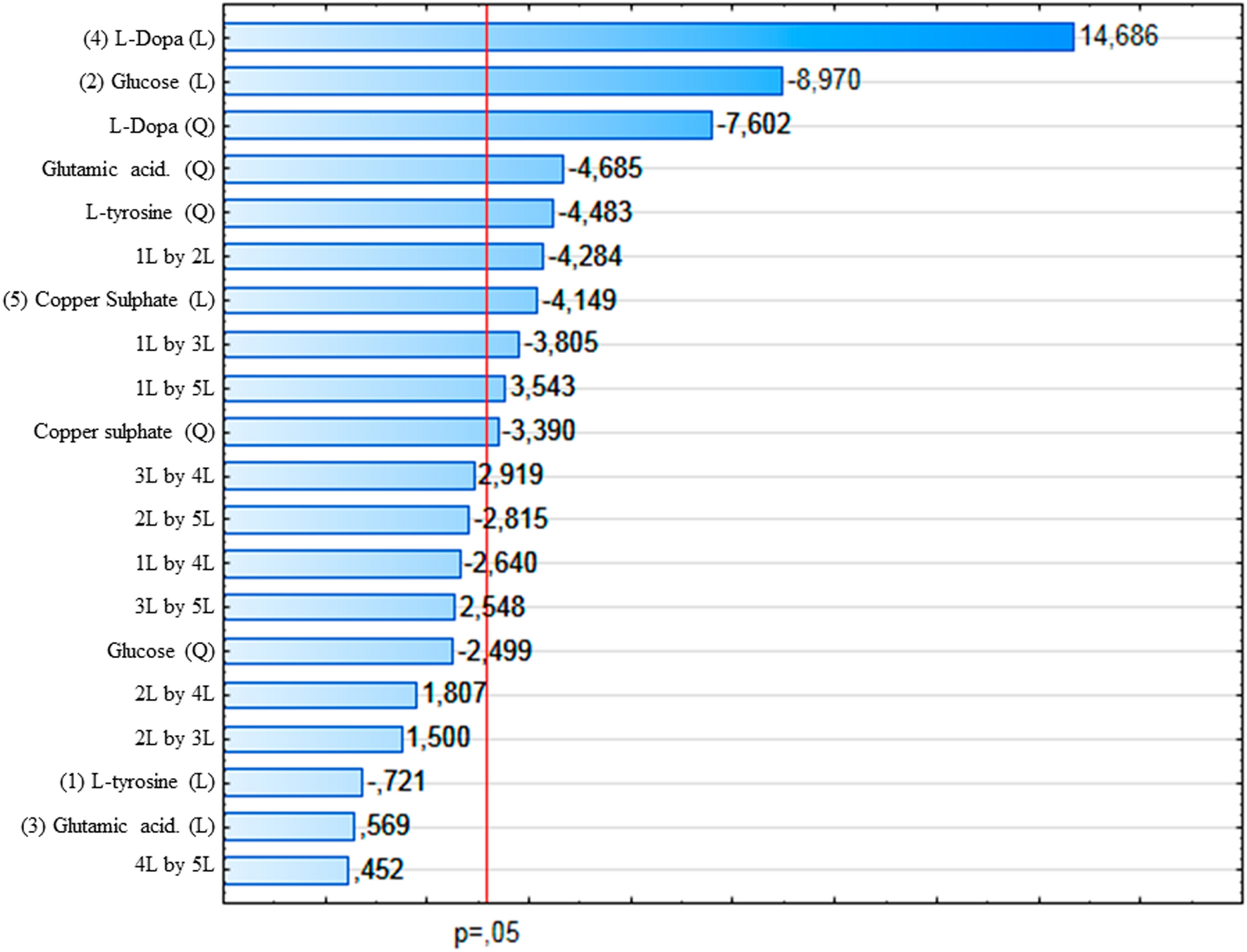
Possibly a big mistake reveling this but here we go
As most of you know melanin turns everything into brown poopo shitskin, it's main function is to provide protection against solar radiation
The same happens in the eye



Eye color is determined by the following process

These melanossomes are within a structure called melanocytes, which migrate to other structures to provide protection


A number of genes are involved in regulating the amount of melanin produced, among which the ones that control the enzyme tyrosinase are the most important

Now, the goal is to apply something that breaks down melanin
Hydrogen peroxide is a natural bleach material, I say natural because it's involved in body functions
A melanin-Cu complex enhances the effect of hydrogen peroxide, but in vivo it might not be achievable or not be ideal
They used a copper nitrate
Copper has been shown to regulate melanin production, in a concentration dependent manner by regulating the enzyme tyrosinase
At lower concentrations it increases melanin biosynthesis but in higher concentrations it seemed to act as a tyrosinase inhibitor, big news if this can be applied in vivo

This might be explained by the fact that melanin binds to metals to protect the body although the authors refer to the concentration of melanin as melanin biosynthesis, so who knows about the underlying process
 link.springer.com
link.springer.com
Now continuing with h2o2
With an alkaline environment the bleaching is highly effective but we have to work at a neutral ph in vivo


I must also mention that the study below says that h2o2 can up the tyrosinase activity by mirroring the quantities of it, not sure who was the nigger that wrote this, idk what that means, but the consequences might be signaling the increase of melanin production
So while h2o2 bleaches in the short term it may cause melanin production to bounce back again

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
Now to show that this shit actually does something this is what happens when you pour 35% h2o2 on your hands
This is likely the result of chemical irritation not removing melanin immediate, I think, maybe it's because of that too

Don't bleach your face with this jfl
H2O2 is normally sold at 3% (v/v) for safety reasons but it causes oxidative damage, obviously
Now for potential eye drops you would want a fraction of that, never 3% or more, the study mentioned concentrations of less than 1mM, I'm not doing the conversions rn but it's likely 1/100 of 3% or less, start low to test if you get eye irritation or something, in vitro is completely different than in vivo, you must go slow and evaluate what's happening to reduce risks.
If someone wants to expand and try something with the copper please be my guest.
YOU SHOULD TRY THIS
THIS COULD BE YOU, ALL THE NEGRALINAS AND POJEETAS YOURS
JUST DROP HYDROGEN PEROXIDE ON YOUR EYEBALLS AND CALL IT A DAY


As most of you know melanin turns everything into brown poopo shitskin, it's main function is to provide protection against solar radiation
The same happens in the eye



This makes the iris the most outer part of the eye that contains melanin, the cornea and anterior chamber are transparentIn the eye, melanin-containing cells are localized in the uveal tract (iris, ciliary body, choroid), and in the retinal pigment epithelium (RPE).
Eye color is determined by the following process
From blue to hazel to brown eye color depends on the level of melanin pigment stored in the melanosome “packets” in the melanocytes of the iris. Blue eyes contain minimal amounts of pigment within a small number of melanosomes. Irises from green–hazel eyes show moderate pigment levels and melanosome number, while brown eyes are the result of high melanin levels stored across many melanosomes

These melanossomes are within a structure called melanocytes, which migrate to other structures to provide protection


A number of genes are involved in regulating the amount of melanin produced, among which the ones that control the enzyme tyrosinase are the most important
The enzyme tyrosinase (TYR) catalyzes the first steps of melanin formation – the formation of l-3,4-dihydroxyphenylalanine (l-DOPA) from the amino acid l-tyrosine as well as dopaquinone from l-DOPA. Defects in the production or function of tyrosinase lead to a decrease or no production of both types of melanins. Studies of two sheep breeds showed that when tyrosinase activity was higher, the coat colors produced were darker and with a greater variety, while a lower level of tyrosinase activity showed the opposite. Albinism is a disorder associated with the mutation of the tyrosinase gene.
Other genes responsible for melanosome and melanin formation are ATP7A, Dct, GPNMB, MLANA, TYRP1, SLC45A2, and RAB38. Mutations in TYR and TYRP1 have been studied extensively due to their role in melanin biosynthesis.

Now, the goal is to apply something that breaks down melanin
Hydrogen peroxide is a natural bleach material, I say natural because it's involved in body functions
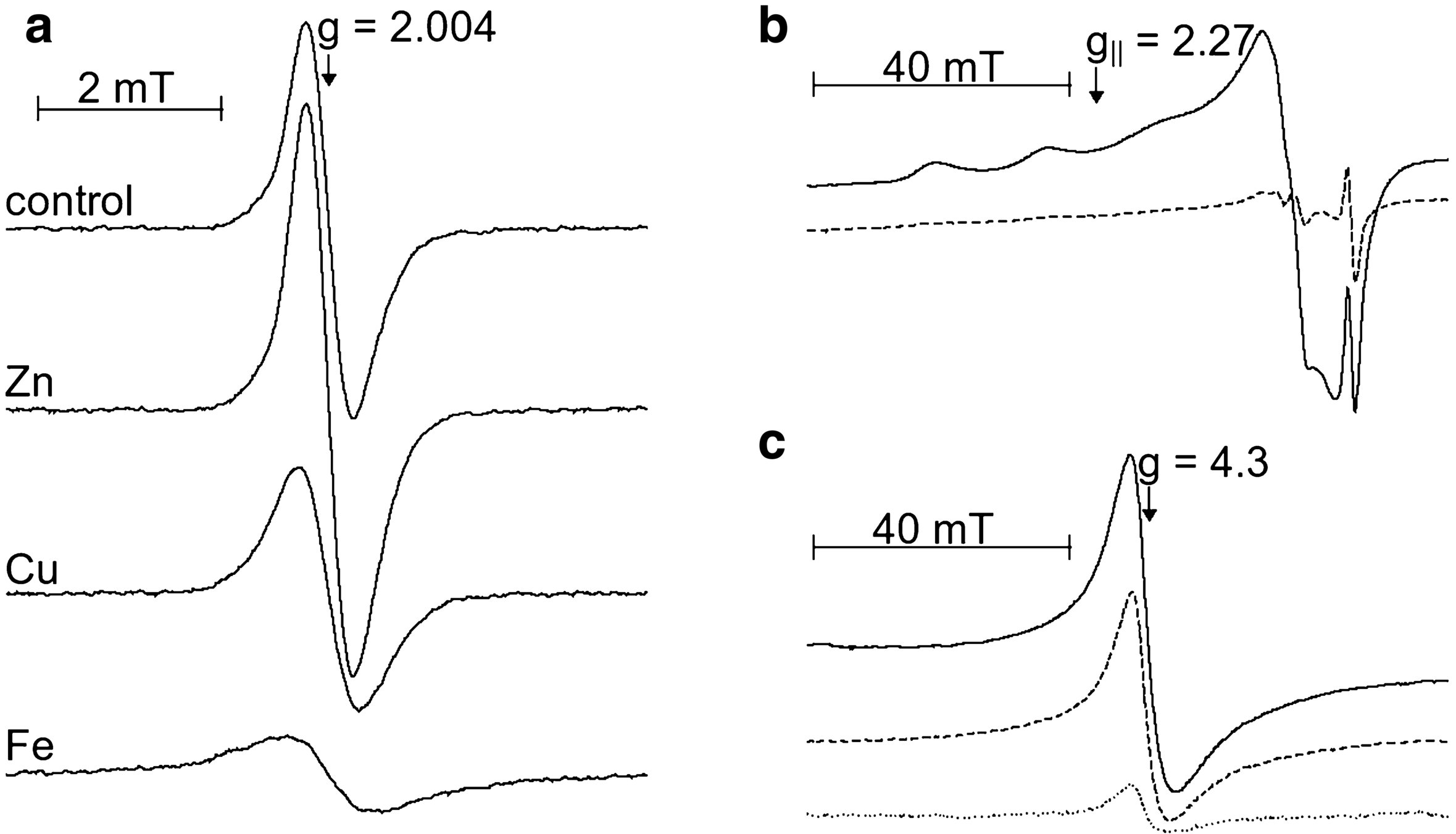
Bleaching of eumelanin has been studied in model systems consisting of synthetic dopa-melanin and various concentrations of hydrogen peroxide, molecular oxygen, and copper(II) ions at neutral and alkaline pH. The data show that at neutral pH, in the dark, metalion-free melanin is very resistant to oxidation by hydrogen peroxide. However, the rate of bleaching of melanin, induced by H202 is significantly accelerated by illumination from UVA-visible light, Bound-to-melanin copper(II) also accelerates the bleaching of melanin with the efficiency dependent on concentration of H202 and oxygen. It suggests possible involvement of melanin-copper complexes in Fenton-like processes. The formation of hydroxyl radicals during melanin bleaching has been concluded on the basis of the electrochemical detection of hydroxylation products of salicylate used as OH scavenger. Redox conversion of bound-to-melanin copper ions was monitored by EPR spectroscopy and direct measurement of melanin Cu(II) complexes. It has been shown that melanin copper(I) complexes were readily oxidized by either oxygen or hydrogen peroxide. The data indicate that bleaching of melanin is a complex process with two distinct stages, reversible oxidation of the hydroquinone moieties of melanin followed by irreversible reactions of the monomers that lead to degradation of the melanin polymer.
A melanin-Cu complex enhances the effect of hydrogen peroxide, but in vivo it might not be achievable or not be ideal
They used a copper nitrate
Copper has been shown to regulate melanin production, in a concentration dependent manner by regulating the enzyme tyrosinase
At lower concentrations it increases melanin biosynthesis but in higher concentrations it seemed to act as a tyrosinase inhibitor, big news if this can be applied in vivo
The tyrosinase cofactor copper greatly enhanced melanin biosynthesis at 5.3 x 10(-6) M, while 1 x 10(-8) M 3,4-dihydroxy-l-phenylalanine hastened pigmentation. The copper-chelating agent KCN and the tyrosinase inhibitor tropolone decreased melanin production at the same concentration of 1 x 10(-5) M

This might be explained by the fact that melanin binds to metals to protect the body although the authors refer to the concentration of melanin as melanin biosynthesis, so who knows about the underlying process
If anyone wants to dig further go ahead, here you might find something(melanin) protects cells from metal toxicity by binding metals (Macaskie and Dean 1991)

Interaction of Melanin with Metal Ions Modulates Their Cytotoxic Potential - Applied Magnetic Resonance
Melanin is one the most common biological pigments. In humans, specialized cells called melanocytes synthesize the pigment from tyrosine and 3,4-dihydroxyphenylalanine via enzyme-catalyzed reactions and spontaneous processes. The formed melanin granule consists of nanoaggregates of oligomers...
Now continuing with h2o2
With an alkaline environment the bleaching is highly effective but we have to work at a neutral ph in vivo
And here are the results both in dark and light conditions with h2o2 and it combined with the copperSynthetic dopa-melanin is a remarkably stable polymer. For instance, we did not observe any changes of optical properties of melanin that was exposed to 0.2 M H202 for several days. In strongly alkaline solution, where ionization of phenolic hydroxyl groups occurs (22), the reactivity of melanin increases and bleaching of the pigment becomes apparent within hours or even minutes of its incubation in H2o2. At pH 9, in 0.15 M H2o2, the absorbance of melanin decreases by 20% within the first 4 h of the incubation, with little consecutive changes (Fig. la). Copper ions, complexed to melanin, accelerated the initial rate of melanin bleaching by a factor of two (Fig. lb).


Now I could go on with explaining a few more things but I already made my pointBleaching of melanin in the dark was compared with light-induced processes. Under the conditions studied, visible and near-UV light enhanced the initial rate of melanin bleaching in 0.15 M H202 by a factor of 10. A common hydroxyl radical scavenger, such as benzoate, at 50 mM concentration, had no effect on melanin photobleaching.
I must also mention that the study below says that h2o2 can up the tyrosinase activity by mirroring the quantities of it, not sure who was the nigger that wrote this, idk what that means, but the consequences might be signaling the increase of melanin production
So while h2o2 bleaches in the short term it may cause melanin production to bounce back again
It is concluded that hydrogen peroxide, formed by the systems, accounts for the elevation of tyrosinase level. When tyrosinase activities determined by 5-S-CD formation were compared to enzyme amounts found by RIA, the ratios of these values were always constant. This fact indicates that the increase in the tyrosinase activities was not due to an activation of the enzyme, but mirrored the quantities of enzyme protein present in the samples. On the basis of our findings, it is assumed that hydrogen peroxide is a regulator of tyrosinase in normal melanocytes and melanoma cells.

Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells - PubMed
The effects of systems generating active oxygen species (superoxide anion, hydrogen peroxide, hydroxyl radical) on tyrosinase have been studied in cultured human melanoma cells. Tyrosinase activity was determined by measuring the quantity of 5-S-L-cysteinyl-L-dopa (5-S-CD) formed in the presence...
Now to show that this shit actually does something this is what happens when you pour 35% h2o2 on your hands
This is likely the result of chemical irritation not removing melanin immediate, I think, maybe it's because of that too

Don't bleach your face with this jfl
H2O2 is normally sold at 3% (v/v) for safety reasons but it causes oxidative damage, obviously
Now for potential eye drops you would want a fraction of that, never 3% or more, the study mentioned concentrations of less than 1mM, I'm not doing the conversions rn but it's likely 1/100 of 3% or less, start low to test if you get eye irritation or something, in vitro is completely different than in vivo, you must go slow and evaluate what's happening to reduce risks.
If someone wants to expand and try something with the copper please be my guest.
YOU SHOULD TRY THIS
THIS COULD BE YOU, ALL THE NEGRALINAS AND POJEETAS YOURS
JUST DROP HYDROGEN PEROXIDE ON YOUR EYEBALLS AND CALL IT A DAY