Deleted member 6403
Made It Out The Hood
- Joined
- Apr 14, 2020
- Posts
- 56,254
- Reputation
- 96,758
Why PUFA is bad: how high membrane polyunsaturation decreases longevity:
This is probably one of the most interesting papers I've read in a long time . It goes into depth about the danger of PUFA and conncects the dots between PUFA ,membranes ,rate of living theory, life expectancy, and cancer .
They state that PUFA increases lipid peroxidation, yet they still manage to conclude that pufa in membranes is good and a lower metabolic rate increases longevity. So the conclusion is off sometimes but the material in this review is invaluable.
I would advise everyone to read the full study. Especially the parts about membrane fatty acid composition! I have copied the parts that I found most interesting and some conclusions.
1. Rate of living theory doesn't adequately explain maximum lifespan
2. Metabolic rate influences cellular memabrane composition
3. More saturated membranes = longer lifespan
4. Even 5%more PUFA in the membrane means 16x more peroxidative damage
5. The carcinogenic /mutagenic lipid peroxidation end-products can ONLY be derived from PUFA
6. Lipid peroxidation (caused by PUFA) is a self reinforcing process
7. PUFA slows down oxidative metabolism by reducing cytochrome oxidase (amongst others)
8. A cornerstone of the rate of living theory is that increasing size in animals equals a lower metabolic rate . This is true for a very simple reason : "If a mouse increased in size to that of a horse and its BMR increased in direct proportion to the increase in body mass, the horse-sized mouse would need a surface temperature of ∼100°C to rid itself of the heat produced by its BMR (134)."
So to have body temperature of 37° (a little more or less in different mammals) the body MUST slow down metabolism ,because otherwise proteins would start to degrade as in high fever. Which does NOT mean that this "slowing down" of metabolism is what's causing longer lifespan in bigger animals !
9. Especially intraspecific studies have shown that there's a positive correlation between metabolic rate and longevity
10. The lower the peroxidation susceptibility (lower PUFA content = lower peroxidation susceptibility) of the liver and muscle membranes the longer the life span of mammals.
"The differences in the characteristic maximum life span of species was initially proposed to be due to variation in mass-specific rate of metabolism. This is called the rate-of-living theory of aging and lies at the base of the oxidative-stress theory of aging, currently the most generally accepted explanation of aging. However, the rate-of-living theory of aging while helpful is not completely adequate in explaining the maximum life span.
Recently, it has been discovered that the fatty acid composition of cell membranes varies systematically between species, and this underlies the variation in their metabolic rate.
When combined with the fact that 1) the products of lipid peroxidation are powerful reactive molecular species, and 2) that fatty acids differ dramatically in their susceptibility to peroxidation, membrane fatty acid composition provides a mechanistic explanation of the variation in maximum life span among animal species.
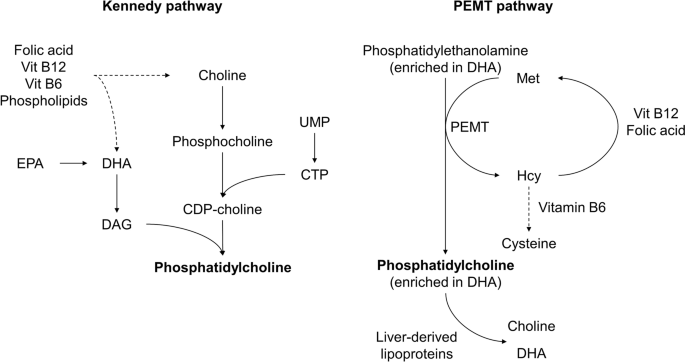
This means that saturated and monounsaturated fatty acyl chains (SFA and MUFA, respectively) are essentially resistant to peroxidation while PUFA are damaged. Furthermore, the greater the degree of polyunsaturation of PUFA, the more prone it is to peroxidative damage. Holman (148) empirically determined (by measurement of oxygen consumption) the relative susceptibilities of the different acyl chains (see Fig. 1). Docosahexaenoic acid (DHA), the highly polyunsaturated omega-3 PUFA with six double bonds, is extremely susceptible to peroxidative attack and is eight times more prone to peroxidation than linoleic acid (LA), which has only two double bonds. DHA is 320 times more susceptible to peroxidation than the monounsaturated oleic acid (OA) (148).
The peroxidation index of a membrane is not the same as its unsaturation index (sometimes also called its “double bond index”), which is a measure of the density of double bonds in the membrane. For example, a membrane bilayer consisting solely of MUFA will have an unsaturation index of 100 and a peroxidation index of 2.5, while a membrane bilayer consisting of 95% SFA and 5% DHA will have an unsaturation index of 30 and a peroxidation index of 40. This means that although the 5% DHA-containing membrane has only 30% the density of double bonds of the monounsaturated bilayer, it is 16 times more susceptible to peroxidative damage.


The resulting peroxyl radical is highly reactive: it can attack membrane proteins and can also oxidize adjacent PUFA chains. Thus the initial reaction is repeated and a free radical chain reaction is propagated. Unless quenched by antioxidants, lipid peroxidation is a self-propagating autocatalytic process producing several potent ROS. It can also generate lipid hydroperoxides (124, 335, 336), which are more hydrophilic than unperoxidized fatty acyl chains, and these can thus disrupt the membrane structure, altering fluidity and other functional properties of membranes.
The hydroperoxides and endoperoxides, generated by lipid peroxidation, can undergo fragmentation to produce a broad range of reactive intermediates, such as alkanals, alkenals, hydroxyalkenals, glyoxal, and malondialdehyde (MDA; Ref. 95) (see Fig. 2). These carbonyl compounds (collectively described as “propagators” in Fig. 2) have unique properties contrasted with free radicals. For instance, compared with ROS or RNS, reactive aldehydes have a much longer half-life (i.e., minutes instead of the microseconds-nanoseconds characteristic of most free radicals). Furthermore, the noncharged structure of aldehydes allows them to migrate with relative ease through hydrophobic membranes and hydrophilic cytosolic media, thereby extending the migration distance far from the production site. On the basis of these features alone, these carbonyl compounds can be more destructive than free radicals and may have far-reaching damaging effects on target sites both within and outside membranes.

These DNA damage markers are mutagenic and carcinogenic, with powerful effects on signal transduction pathways (217).
Furthermore, they 1) are present in the genome of healthy humans, and other animal species, at biologically significant levels (similar or even higher than oxidation markers sensu stricto) (55), 2) are efficient inducers of mutations frequently detected in oncogenes or tumor suppressor genes from human tumors (254), 3) show increased levels in aged animals (55), 4) can be repaired by nucleotide excision repair systems and metabolized by oxidative pathways (262), 5) correlate with alterations in cell cycle control and gene expression in cultured cells (169), and 6) increase nearly 20-fold with a high-PUFA diet (97).
Thus lipid peroxidation should not be perceived solely in a “damage to lipids” scenario, but should also be considered as a significant endogenous source of damage to other cellular macromolecules, such as proteins and DNA (including mutations). In this way, variation in membrane fatty acid composition, by influencing lipid peroxidation, can have significant effects on oxidative damage to many and varied cellular macromolecules. For example, peroxidized cardiolipin in the mitochondrial membrane can inactivate cytochrome oxidase by mechanisms both similar to hydrogen peroxide and also mechanisms unique to organic hydroperoxides (251).
The variation obvious in Figure 6 is a clear demonstration that the rate-of-living generalization is only a rough predictor of how long a mammal species can maximally live. Its inability to precisely describe the maximum longevity of a mammal suggests other factors are involved in the determination of maximum life span.
"Intraspecific studies on dogs (333), mice (234, 332), and humans (301) reveal a positive association between maximum life span and mass-specific metabolic rate "
Several intraspecific studies using mice and rats (40, 146, 202, 332, 333) have not observed an inverse relationship between mass-specific metabolic rate and MLSP. Indeed, some of these studies show the opposite of rate-of-living predictions, namely, that mice with high mass-specific metabolic rates tend to live longer than those individuals with low metabolic rates.
The liver mitochondrial membrane PI of mammals is proportional to their MLSP−0.40, which means that a 24% decrease in their peroxidative susceptibility is associated with every doubling of maximum life span. For skeletal muscle membranes, the corresponding value is that a 19% decrease in peroxidative susceptibility is associated with every doubling of MLSP in mammals (i.e., muscle PI is proportional to MLSP−0.30).

For example, if fed a diet devoid of PUFA, mammals will synthesize an unusual PUFA, mead acid (20:3 n-9) and accumulate it, together with more than normal amounts of MUFA in their membranes. However, with extreme manipulation of dietary fat composition, it is possible to effect small changes in membrane fat composition.
A low PUFA content in cellular membranes (and particularly in the inner mitochondrial membrane) will be advantageous in decreasing the sensitivity of the membrane to lipid peroxidation and would consequently also protect other molecules against lipoxidation-derived damage.
The studies summarized in Table 5 show that there are many reports of 1) an increase in either PUFA content or PI of membranes with age, 2) an increase in both in vitro and in vivo membrane lipid peroxidation with age, as well as 3) age-related changes in physicochemical membrane properties.
In view of these widespread changes in membrane composition and lipid peroxidation with age, it is of interest that in the senescence-accelerated mouse (SAM) strain, those mice that are SAM-prone (SAM-P mice) have greater levels of the highly polyunsaturated peroxidation-prone fatty acids (both 22:6 n-3 and 20:4 n-6) and lower levels of the less peroxidation-prone PUFA (18:2 n-6) in their membranes, and consequently a greater PI, than SAM-resistant mice (59, 281). SAM-prone mice also show greater degrees of lipid peroxides in their tissues than do SAM-resistant mice (221).
Regardless of the factors ultimately responsible for MLSP variation, there are two traits that are often associated with long-lived species: reduced rates of mitochondrial free radical production and reduced susceptibility of membranes to lipoxidation."
https://journals.physiology.org/doi/full/10.1152/physrev.00047.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
This is probably one of the most interesting papers I've read in a long time . It goes into depth about the danger of PUFA and conncects the dots between PUFA ,membranes ,rate of living theory, life expectancy, and cancer .
They state that PUFA increases lipid peroxidation, yet they still manage to conclude that pufa in membranes is good and a lower metabolic rate increases longevity. So the conclusion is off sometimes but the material in this review is invaluable.
I would advise everyone to read the full study. Especially the parts about membrane fatty acid composition! I have copied the parts that I found most interesting and some conclusions.
1. Rate of living theory doesn't adequately explain maximum lifespan
2. Metabolic rate influences cellular memabrane composition
3. More saturated membranes = longer lifespan
4. Even 5%more PUFA in the membrane means 16x more peroxidative damage
5. The carcinogenic /mutagenic lipid peroxidation end-products can ONLY be derived from PUFA
6. Lipid peroxidation (caused by PUFA) is a self reinforcing process
7. PUFA slows down oxidative metabolism by reducing cytochrome oxidase (amongst others)
8. A cornerstone of the rate of living theory is that increasing size in animals equals a lower metabolic rate . This is true for a very simple reason : "If a mouse increased in size to that of a horse and its BMR increased in direct proportion to the increase in body mass, the horse-sized mouse would need a surface temperature of ∼100°C to rid itself of the heat produced by its BMR (134)."
So to have body temperature of 37° (a little more or less in different mammals) the body MUST slow down metabolism ,because otherwise proteins would start to degrade as in high fever. Which does NOT mean that this "slowing down" of metabolism is what's causing longer lifespan in bigger animals !
9. Especially intraspecific studies have shown that there's a positive correlation between metabolic rate and longevity
10. The lower the peroxidation susceptibility (lower PUFA content = lower peroxidation susceptibility) of the liver and muscle membranes the longer the life span of mammals.
"The differences in the characteristic maximum life span of species was initially proposed to be due to variation in mass-specific rate of metabolism. This is called the rate-of-living theory of aging and lies at the base of the oxidative-stress theory of aging, currently the most generally accepted explanation of aging. However, the rate-of-living theory of aging while helpful is not completely adequate in explaining the maximum life span.
Recently, it has been discovered that the fatty acid composition of cell membranes varies systematically between species, and this underlies the variation in their metabolic rate.
When combined with the fact that 1) the products of lipid peroxidation are powerful reactive molecular species, and 2) that fatty acids differ dramatically in their susceptibility to peroxidation, membrane fatty acid composition provides a mechanistic explanation of the variation in maximum life span among animal species.
This means that saturated and monounsaturated fatty acyl chains (SFA and MUFA, respectively) are essentially resistant to peroxidation while PUFA are damaged. Furthermore, the greater the degree of polyunsaturation of PUFA, the more prone it is to peroxidative damage. Holman (148) empirically determined (by measurement of oxygen consumption) the relative susceptibilities of the different acyl chains (see Fig. 1). Docosahexaenoic acid (DHA), the highly polyunsaturated omega-3 PUFA with six double bonds, is extremely susceptible to peroxidative attack and is eight times more prone to peroxidation than linoleic acid (LA), which has only two double bonds. DHA is 320 times more susceptible to peroxidation than the monounsaturated oleic acid (OA) (148).
The peroxidation index of a membrane is not the same as its unsaturation index (sometimes also called its “double bond index”), which is a measure of the density of double bonds in the membrane. For example, a membrane bilayer consisting solely of MUFA will have an unsaturation index of 100 and a peroxidation index of 2.5, while a membrane bilayer consisting of 95% SFA and 5% DHA will have an unsaturation index of 30 and a peroxidation index of 40. This means that although the 5% DHA-containing membrane has only 30% the density of double bonds of the monounsaturated bilayer, it is 16 times more susceptible to peroxidative damage.


The resulting peroxyl radical is highly reactive: it can attack membrane proteins and can also oxidize adjacent PUFA chains. Thus the initial reaction is repeated and a free radical chain reaction is propagated. Unless quenched by antioxidants, lipid peroxidation is a self-propagating autocatalytic process producing several potent ROS. It can also generate lipid hydroperoxides (124, 335, 336), which are more hydrophilic than unperoxidized fatty acyl chains, and these can thus disrupt the membrane structure, altering fluidity and other functional properties of membranes.
The hydroperoxides and endoperoxides, generated by lipid peroxidation, can undergo fragmentation to produce a broad range of reactive intermediates, such as alkanals, alkenals, hydroxyalkenals, glyoxal, and malondialdehyde (MDA; Ref. 95) (see Fig. 2). These carbonyl compounds (collectively described as “propagators” in Fig. 2) have unique properties contrasted with free radicals. For instance, compared with ROS or RNS, reactive aldehydes have a much longer half-life (i.e., minutes instead of the microseconds-nanoseconds characteristic of most free radicals). Furthermore, the noncharged structure of aldehydes allows them to migrate with relative ease through hydrophobic membranes and hydrophilic cytosolic media, thereby extending the migration distance far from the production site. On the basis of these features alone, these carbonyl compounds can be more destructive than free radicals and may have far-reaching damaging effects on target sites both within and outside membranes.

These DNA damage markers are mutagenic and carcinogenic, with powerful effects on signal transduction pathways (217).
Furthermore, they 1) are present in the genome of healthy humans, and other animal species, at biologically significant levels (similar or even higher than oxidation markers sensu stricto) (55), 2) are efficient inducers of mutations frequently detected in oncogenes or tumor suppressor genes from human tumors (254), 3) show increased levels in aged animals (55), 4) can be repaired by nucleotide excision repair systems and metabolized by oxidative pathways (262), 5) correlate with alterations in cell cycle control and gene expression in cultured cells (169), and 6) increase nearly 20-fold with a high-PUFA diet (97).
Thus lipid peroxidation should not be perceived solely in a “damage to lipids” scenario, but should also be considered as a significant endogenous source of damage to other cellular macromolecules, such as proteins and DNA (including mutations). In this way, variation in membrane fatty acid composition, by influencing lipid peroxidation, can have significant effects on oxidative damage to many and varied cellular macromolecules. For example, peroxidized cardiolipin in the mitochondrial membrane can inactivate cytochrome oxidase by mechanisms both similar to hydrogen peroxide and also mechanisms unique to organic hydroperoxides (251).
The variation obvious in Figure 6 is a clear demonstration that the rate-of-living generalization is only a rough predictor of how long a mammal species can maximally live. Its inability to precisely describe the maximum longevity of a mammal suggests other factors are involved in the determination of maximum life span.
"Intraspecific studies on dogs (333), mice (234, 332), and humans (301) reveal a positive association between maximum life span and mass-specific metabolic rate "
Several intraspecific studies using mice and rats (40, 146, 202, 332, 333) have not observed an inverse relationship between mass-specific metabolic rate and MLSP. Indeed, some of these studies show the opposite of rate-of-living predictions, namely, that mice with high mass-specific metabolic rates tend to live longer than those individuals with low metabolic rates.
The liver mitochondrial membrane PI of mammals is proportional to their MLSP−0.40, which means that a 24% decrease in their peroxidative susceptibility is associated with every doubling of maximum life span. For skeletal muscle membranes, the corresponding value is that a 19% decrease in peroxidative susceptibility is associated with every doubling of MLSP in mammals (i.e., muscle PI is proportional to MLSP−0.30).

For example, if fed a diet devoid of PUFA, mammals will synthesize an unusual PUFA, mead acid (20:3 n-9) and accumulate it, together with more than normal amounts of MUFA in their membranes. However, with extreme manipulation of dietary fat composition, it is possible to effect small changes in membrane fat composition.
A low PUFA content in cellular membranes (and particularly in the inner mitochondrial membrane) will be advantageous in decreasing the sensitivity of the membrane to lipid peroxidation and would consequently also protect other molecules against lipoxidation-derived damage.
The studies summarized in Table 5 show that there are many reports of 1) an increase in either PUFA content or PI of membranes with age, 2) an increase in both in vitro and in vivo membrane lipid peroxidation with age, as well as 3) age-related changes in physicochemical membrane properties.
In view of these widespread changes in membrane composition and lipid peroxidation with age, it is of interest that in the senescence-accelerated mouse (SAM) strain, those mice that are SAM-prone (SAM-P mice) have greater levels of the highly polyunsaturated peroxidation-prone fatty acids (both 22:6 n-3 and 20:4 n-6) and lower levels of the less peroxidation-prone PUFA (18:2 n-6) in their membranes, and consequently a greater PI, than SAM-resistant mice (59, 281). SAM-prone mice also show greater degrees of lipid peroxides in their tissues than do SAM-resistant mice (221).
Regardless of the factors ultimately responsible for MLSP variation, there are two traits that are often associated with long-lived species: reduced rates of mitochondrial free radical production and reduced susceptibility of membranes to lipoxidation."
https://journals.physiology.org/doi/full/10.1152/physrev.00047.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org